Linear Dichroism
Why do we use linear dichroism?
Linear Dichroism (LD) is a spectroscopic technique that can be used with systems that are either intrinsically oriented, or can be oriented during an experiment by external forces. It gives information about conformation and orientation of structures within molecules.
Knowing the structure of a molecule is one of the keys to deducing its function in a biological system. Many biomacromolecules, however, are not amenable to structural characterisation by powerful techniques such as NMR and X-ray diffraction because they are too large, too flexible or cannot be crystallized. Long molecules such as
DNA, DNA-ligand complexes, DNA-enzyme complexes
Fibrous proteins including cytoskeletal proteins, Alzheimers and prion proteins, collagen
Membrane proteins and peptides in liposomes (which are distorted by the flow so acquiring a high enough aspect ratio to orient)
Carbon nanotube, carbon nanotube-ligand complexes
LD is also ideal for following the kinetics of any reaction which involves changing the structure or length of a molecule. A simple example is that of restriction enzyme activity on DNA.
What is linear dichroism?
To measure LD the sample is oriented, then the difference in absorption of light linearly polarised parallel and perpendicular to the orientation axis is measured
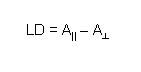
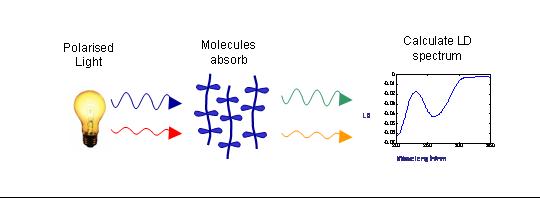
In the case of flow orientation of long molecules the orientation axis is the long axis of the molecule. The figure below shows a schematic of how LD is measured.
We have designed the Couette flow LD cells we use in our research. The refinements of our original designs are now made by Diotica Scientific Ltd. and sold by Kromatek http://www.kromatek.co.uk/categories/Linear-Dichroism-Couettes
Representative publications
Rodger, A.; Nordén, B. “Circular dichroism and linear dichroism”; Oxford University Press, 1997, pp150 (unfortunately this is out of print, but we are working on a new edition)
Halsall, D.J.; Dafforn, T.R; Marrington, R.; Halligan, E.; and Rodger, A. “Linear Dichroism for quantitative Polymerase Chain Reaction applications: An intrinsic signal from DNA has the potential to be an interference-free, specific and label free probe for real-time and quantitative PCR” IVD Technology, 2004, 6, 51-60

A Couette flow cell used for LD.
The light beam comes from the left and is collected by a photomultiplier tube on the right, having passed through the sample.
Below is a sample holder and rod.

