Metals in Medicine: Research
Photoactivated Anticancer Complexes |
|||||
|
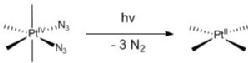
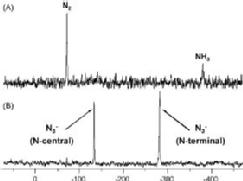
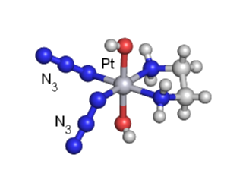
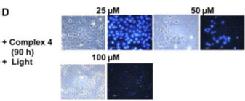
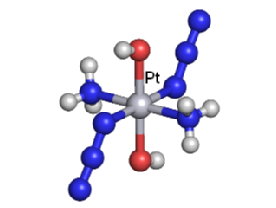
We are exploring the use of inert, nontoxic platinum complexes that can be activated locally in cancer cells by light. Our research on such photoactivated metallo-anticancer agents focuses on Pt(IV) complexes and makes use of the fact that they are kinetically inert under biological conditions and can be reduced to potentially reactive Pt(II) complexes upon irradiation. |
 |
||||
Photoactivated Ruthenium(II)-arene anticancer complexes
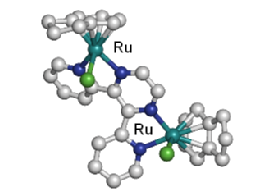
Recently, we have also become interested in extending our family of ruthenium-arene anticancer complexes to organometallic complexes that can be photoactivated. The first results showed that dinuclear Ru(II)-arene complexes [ |
Dinuclear Ru(II)-arene complexes can be photoactivated to produce highly reactive Ru(II) species |
|
| Selection of our recent publications on our photoactivated anticancer complexes: | |||
| 1. | Bednarski PJ, Mackay FS, Sadler PJ: Photoactivatable Platinum Complexes. Anti-Cancer Agents in Medicinal Chemistry 2007, 7:75-93. | ||
| 2. | Kratochwil NA, Parkinson JA, Bednarski PJ, Sadler PJ: Nucleotide platination induced by visible light. Angewandte Chemie-International Edition 1999, 38:1460-1463. | ||
| 3. | Müller P, Schröder B, Parkinson JA, Kratochwil NA, Coxall RA, Parkin A, Parsons S, Sadler PJ: Nucleotide cross-linking induced by photoreactions of platinum(IV)-azide complexes. Angewandte Chemie-International Edition 2003, 42:335-339. | ||
| 4. | Kasparkova J, Mackay FS, Brabec V, Sadler PJ: Formation of platinated GG cross-links on DNA by photoactivation of a platinum(IV) azide complex. Journal of Biological Inorganic Chemistry 2003, 8:741-745. | ||
| 5. | Ronconi L, Sadler PJ: Unprecendented carbon-carbon bond formation induced by photoactivation of a platinum(IV)-diazido complex. Chemical Communications 2008:235-237. | ||
| 6. | Bednarski PJ, Grunert R, Zielzki M, Wellner A, Mackay FS, Sadler PJ: Light-activated destruction of cancer cell nuclei by platinum diazide complexes. Chemistry & Biology 2006, 13:61-67. | ||
| 7. | Heringova P, Woods J, Mackay FS, Kasparkova J, Sadler PJ, Brabec V: Transplatin is cytotoxic when photoactivated: Enhanced formation of DNA cross-links. Journal of Medicinal Chemistry 2006, 49:7792-7798. | ||
| 8. | Mackay FS, Woods JA, Moseley H, Ferguson J, Dawson A, Parsons S, Sadler PJ: A photoactivated trans-diammine platinum complex as cytotoxic as cisplatin. Chemistry-a European Journal 2006, 12:3155-3161. | ||
| 9. | Mackay FS, Woods JA, Heringova P, Kasparkova J, Pizarro AM, Moggach SA, Parsons S, Brabec V, Sadler PJ: A potent cytotoxic photoactivated platinum complex. Proc. Natl. Acad. Sci. USA 2007, 104:20743-20748 |
||
| 10. | Magennis SW, Habtemariam A, Novakova O, Henry JB, Meier S, Parsons S, Oswald IDH, Brabec V, Sadler PJ: Dual triggering of DNA binding and fluorescence via photoactivation of a dinuclear ruthenium(II) arene complex. Inorganic Chemistry 2007, 46:5059-5068. | ||