Electrocatalysis
IntroductionElectrocatalysts are of ubiquitous importance and can be found in a large array of research fields and applications, including corrosion science, development of electroanalytical sensors, waste water treatment, electro-organic synthesis and energy conversion devices (e.g. batteries, fuel cells and solar cells). To optimise the utilisation of electrocatalytic materials (often noble metals), the catalytic material is often dispersed as nanoparticles (or other nanomaterials) on a conducting support material. Consequently, the performance of an electrocatalytic ensemble towards a particular reaction or application is the result of an interplay between a large number of factors, including the nature of the electrocatalytic particles (materials, size, shape, surface structure), the nature of the support (material, surface structure, electron transfer properties) and the interaction between the catalytic particles and the support material. |
 |
|
In the Electrochemistry and Interfaces group at Warwick, we study this subtle interplay and aim to gain a deeper insight in the role of the various factors on the overall performance by combining electrochemical techniques with high resolution microscopy techniques and electrochemical scanning probe techniques. In addition, we develop novel methods to prepare and study electrocatalytic systems. |
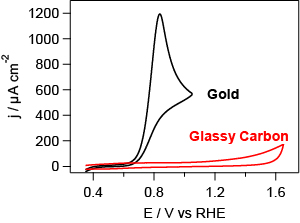
Oxidation of 2 mM hydrazine (N2H4) in 50 mM K2SO4 + 0.5 mM KHSO4 illustrating the catalytic effect of gold. Scan rate 50 mV s-1 |
|
New catalytic systemsThe activity of an electrocatalyst is a complex interplay between many factors, such as the morphology and electronic structure of the catalytic material, and the nature of the support material. In addition, different reactions will have different requirements for the catalytic system. Consequently, there is no general approach for optimising catalytic systems, and every reaction requires a specifically tailored catalyst. In the Electrochemistry and Interfaces group at Warwick, we prepare and study novel catalytic systems towards various reactions. |
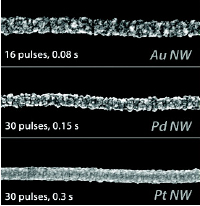 |
| Catalytic nanowires prepared by pulsed electrodeposition onto a single walled carbon nanotube.(ACS Nano, 2011, 5, 10017-10025.) |
(Towards) Single particle catalysisThe relationship between the structural properties, such as the size, shape and surface structure of a catalytic nanoparticle and its reactivity is a key concept in electrocatalysis. The current understanding of this relationship is mostly derived from investigations employing large ensembles of nanoparticles. However, only average properties can be derived from ensemble studies, and the reactivity of individual particles can be obscured by this averaging. In addition, some electrocatalytic reactions are sensitive to interparticle spacing, due to overlapping diffusion fields, which is difficult to control in ensemble studies. Consequently, the information that can be obtained by ensemble studies is necessarily limited, and deriving fundamental structure-reactivity relationships from such studies is challenging. In order to circumvent this limitations and to elucidate the true relationship between structure and reactivity, it is necessary to move from ensemble studies towards studies involving single particles. However, such studies are complicated by challenges of locating a single particle, isolating it from surrounding particles, measuring the small resultant signal and characterising the particles. In the Electrochemistry and Interfaces group at Warwick, we are developing novel techniques and methods to tackle these challenges. An example of our work on single particles is shown below. |
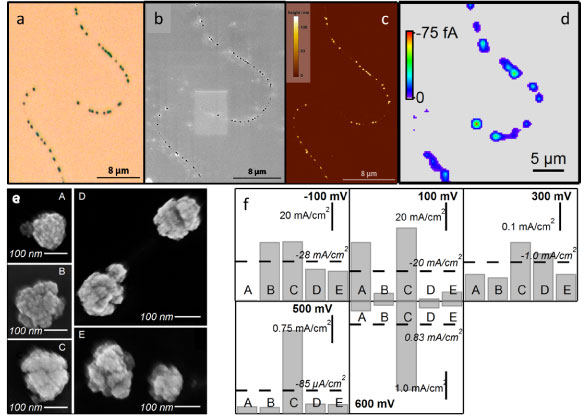 |
| Studying reactivity of single platinum nanoparticles in an ensemble supported on a carbon nanotube. (a) Optical microscopy image. (b) Field emission – scanning electron microscopy (FE-SEM) image. (c) Atomic force microscopy (AFM) image. (d) Scanning electrochemical cell microscopy (SECCM) image: the electrochemical activity of single particles can clearly be resolved. Particles held at 500 mV vs. Pd-H2 to drive oxygen reduction, solution: 0.5 M H2SO4 (aerated). (e) High resolution FE-SEM images of selected (groups of) particles. (f) Current density plots for the selected (groups of) particles at different potentials. (J. Am. Chem. Soc., 2011, 133, 10744-10747, highlighted in Nature Materials) |
PhotocatalysisAs a branch of electrocatalysis, photoelectrocatalysis involves the study of electrochemical processes catalysed by light sensitive electrodes. Photoelectrocatalysis is the essence of all solar energy applications and is the process that drives photosynthesis in plants. In our group, high resolution imaging techniques are employed to study the processes occurring at photosensitive electrodes on the submicron scale. In particular, we are currently studying electrode processes occurring in dye sensitised solar cells (DSSCs). |
|
Dye sensitised solar cells (DSSC) |
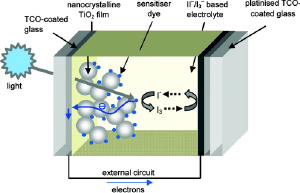 Schematic of the components and mechanism of a typical dye sensitised solar cell (DSSC) where TiO2 is the semiconductor and the triiodide/iodide couple is the mediator. Both electrodes are supported on a transparent conducting oxide (TCO) glass electrode. Adapted from Chem Mater, 2011, 5, 3381-3399. |
