Nanotubes & Graphene
- Single Carbon Nanotube
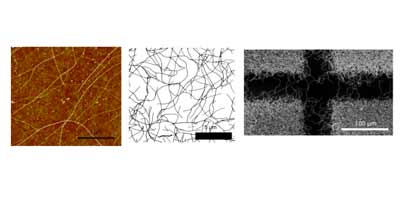
- Carbon Nanotube Networks
- Aligned Carbon Nanotubes
- Vertically Aligned SWNT
- Graphene
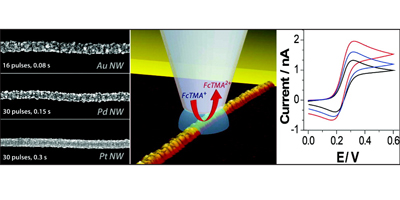
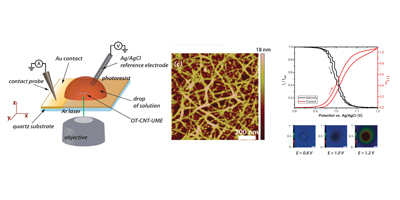
In conjunction with micro and smaller sized capillaries filled with the analyte of interest it is possible to elucidate the electrochemical activity of different redox species, at individual SWNTs, and also electrodeposit metals to form nanoparticle-nanotube composites or metallic nanowires. Our results show that the sidewall of a SWNT supports fast electron transport.
Related References:
|
|
|
|
|
|
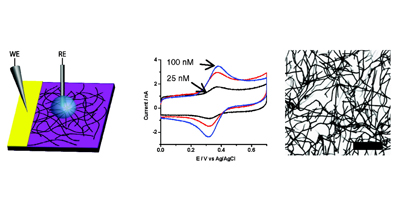
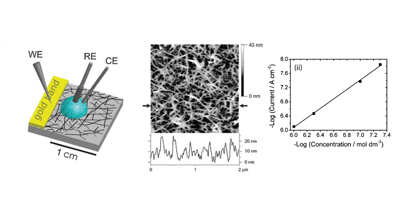
Randomly oriented SWNTs grown by CVD on an insulator substrate have demonstrated overwhelming suitability as an electrochemical sensor, enabling nM detection using CV alone in stationary solution! This is due to the fact that less than 1 % of the surface is covered in SWNTs and the SWNTs have a low intrinsic capacitance. Fundamental studies of electron transfer at SWNT networks has enabled the electroactivity to be mapped using SECCM along the entire length of a SWNT, again displaying high electroactivity. These types of SWNT ensemble have also been evaluated for electrical applications and as a transparent electrode.
Related References:
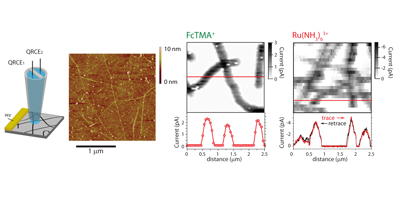
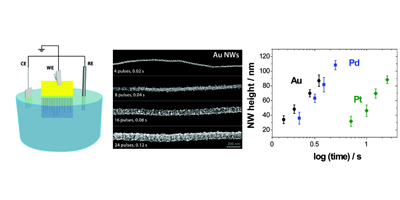
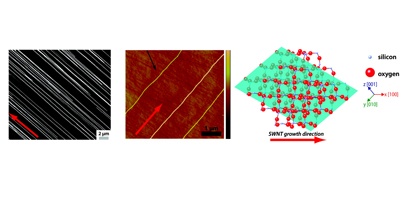
Aligned nanotubes enable the fabrication of single tube carbon nanotube devices. Alignment can be achieved by either epitaxial growth or gas flow alignment. The mechanism of alignment and the electrochemical properties of aligned SWNTs (at the single SWNT level) have been investigated. Aligned SWNTs have also been used as templates for the electrodeposition of nanowires and nanoparticles.
Related References:
|
|
|
|
|
|
|
|
|
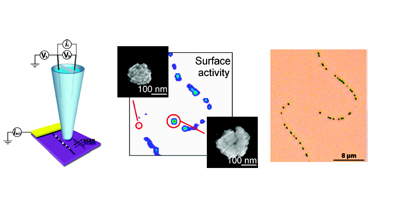
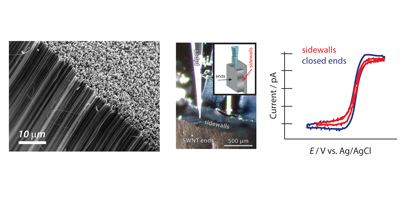
These 3 dimensional structures, also known as forests, consist of a high density of vertically aligned SWNTs. In our group we have been working on their synthesis in order to optimize the quality and the compactness of this type of carbon nanotube arrangement. An important characteristic of this material is the ability to access independently nanotube ends and sidewalls. This has been utilised to study the fundamentals of the electron transfer of each site, and conclude that both are of equal high electrochemical activity. These findings raise new possibilities in the design of CNT forests as sensing device.
Related References:
|
|
|
|
|
|
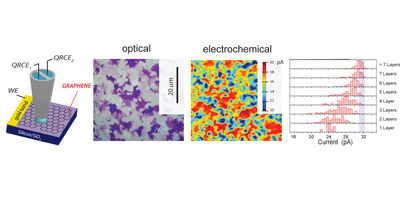
Graphene, this one-atom thick layer of sp2 carbon, is currently one of the most studied materials. In our laboratory we produce graphene using both atmospheric pressure CVD and low pressure CVD; Ni catalysts result in multilayered flakes, whilst Cu allows for a continuous single layer graphene film. Those materials have been studied in terms of electron transfer capability concluding that the electron transfer capabilties of the material increases as the number of graphene layers increases. These are important implications in the design of graphene based devices for enhance electrochemical performance.
Related References:
|
|
|