Wills Group: Hydrogen Energy Research at Warwick
Funded by AWM Science cities investment. Full details of the Birmingham/Warwick joint programme are here.
Project lead at Warwick: Professor Martin Wills, Chemistry
Background:
The hydrogen energy project is one of two components of the broader ‘Energy Futures’ project, which is one of the key areas funded under Science Cities. The other component is ‘Energy Efficiency’.
The programme’s Core Projects and funding sources:
Infrastructure funding of ca. £6.3M from Advantage West Midlands has been invested in several departments in the Universities of Birmingham and Warwick. The hydrogen energy project, which started in January 2007, has already led to the establishment of several interdisciplinary research collaborations between scientists at Warwick and Birmingham, to the leverage of further funds through successful grant applications, and to the submission of further applications for funding from research councils, the EU, industry and charities.
The work on the hydrogen energy project can be broadly characterised into three areas;
1) New methods for hydrogen generation:
Hydrogen generation from biomass is being studied at Warwick HRI using a new solid biomass fermenter (David Pink, Kerry Burton, Guy Barker, Simon Bright) and HPLC analysis system purchased with Science Cities funds. Researchers from Biological Science (Paul Norris) and Chemistry (Tim Bugg) are also participating in this project.


Chemical catalysis of hydrogen generation is being developed in the department of chemistry at Warwick (Martin Wills) in collaboration with Chemical Engineering at Birmingham. An application has been made to the BBSRC ‘Bioenergy’ call for programme grants and has been shortlisted.
A graph showing hydrogen generation (litres) from formic acid
using 14mg of a Ru catalyst at 100 degrees:
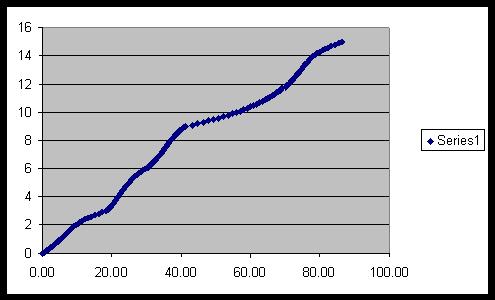
2) Hydrogen storage – methodology and characterisation.
The department of Physics (Mark Smith, Pam Thomas) are investigating solid-state hydrogen storage systems based on metal hydrides using a combination of Nuclear Magnetic Resonance and low-angle X-ray diffraction techniques; two new instruments have been purchased with Science cities funds for this application.


The department of Chemistry (Patrick Unwin, Julie Macpherson) are also involved in the study and characterisation of hydrogen storage media and have acquired a new state of the art instruments including a scanning electrochemical microscope, light microscope and biopotentiostat from Science Cities funds.
3) Energy from hydrogen using fuel cells.
This component of the project is largely focussed on Birmingham, however there are now several collaborations in place between both institutes - full details are here.
Equipment made available at both Warwick and Birmingham funded by Science cities:
Solid state X-ray diffractometers, mass spectrometers, Solid state NMR.
FTIR, / RAMAN, Glove boxes, Confocal Microscopy.
High pressure reaction cells, pressure vessels,
Fuel cells, large scale bioreactors, biohydrogen pilot plant.
Extensive analytical equipment, Anaerobic growth reactors.
Scanning electrochemical microscope and associated equipment.
Further collaborations resulting from Hydrogen Energy project investment:
i) between Scientists at Warwick HRI and Biological sciences and the Birmingham University departments of chemical engineering and biochemistry – BBSRC ‘Bioenergy’ programme grant shortlisted.
ii) Application to BBSRC Bioenergy call on hydrogen from biomass shortlisted (value £2.5M, two researchers from Warwick and two from Birmingham).
iii) Successful EPSRC ‘feasibility study’ grant to Warwick Physics/Birmingham Biochemistry awarded.
iv) Birmingham/Warwick academics in further collaborative bids for Fuel Cell and Biomass conversion grants.
|
|
Hydrogen gas, 200atm |
Liquid hydrogen (0.07kg/L @ 20K)
|
Glucose
C6H12O6
|
Material containing 6% H by mass
|
Hexane C6H14 (0.67 kg/L)
|
|
Moles H2 per kg
|
-
|
500
|
33.3
|
25
|
-
|
|
Moles H2 per litre
|
8.33
|
35
|
-
|
-
|
-
|
|
Energy/ kJ/mol
|
237.2
|
237.2
|
237.2 (1)
|
237.2 (1)
|
4,017 (2)
|
|
Energy/ kJ per kg
|
-
|
118,600 (14.2L)
|
7,898 (1)
|
7,116 (1)
|
46,711 (2)
|
|
Energy/ kJ per L
|
1,975
|
8,302
|
-
|
-
|
31,296 (2)
|
