Researchers apply computing power to crack egg shell problem
 Researchers at the University of Warwick and the University of Sheffield have applied computing power to crack a problem in egg shell formation. The work may also give a partial answer to the age old question “what came first the chicken or the egg?”
Researchers at the University of Warwick and the University of Sheffield have applied computing power to crack a problem in egg shell formation. The work may also give a partial answer to the age old question “what came first the chicken or the egg?”
The answer to the question in this context is “chicken” or – at least a particular chicken protein. There is however a further twist in that this particular chicken protein turns out to come both first and last. That neat trick it performs provides new insights into control of crystal growth which is key to egg shell production.
Researchers had long known that a chicken eggshell protein called ovocleidin-17 (OC-17) must play some role in egg shell formation. The protein is found only in the mineral region of the egg (the hard part of the shell) and lab bench results showed that it appeared to influence the transformation of (CaCo3) into calcite crystals. The mechanism of this control remained unclear. How this process could be used to form an actual eggshell remained unclear.
University of Warwick researchers Mark Rodger and David Quigley, in collaboration with colleagues at the University of Sheffield, have now been able to apply a powerful computing tool called metadynamics and the UK national supercomputer in Edinburgh to crack this egg problem.
 Dr David Quigley from the Department of Physics and Centre for Scientific Computing, University of Warwick, said: “Metadynamics extends conventional molecular dynamics (MD) simulations and is particularly good at sampling transitions between disordered and ordered states of matter.”
Dr David Quigley from the Department of Physics and Centre for Scientific Computing, University of Warwick, said: “Metadynamics extends conventional molecular dynamics (MD) simulations and is particularly good at sampling transitions between disordered and ordered states of matter.”
Using these tools The Warwick and Sheffield researchers were able to create simulations that showed exactly how the protein bound to amorphous calcium carbonate surface using two clusters of “arginine residues”, located on two loops of the protein and creating a literal chemical “clamp” to nano sized particles of calcium carbonate.
While clamped in this way, the OC-17 encourages the nanoparticles of calcium carbonate to transform into “calcite crystallites” that form the tiny of nucleus of crystals that can continue to grow on their own. But they also noticed that sometimes this chemical clamp didn’t work. The OC-17 just seemed to detatch from the nanoparticle or “be desorbed”.
Professor Mark Rodger from Department of Chemistry and Centre for Scientific Computing, University of Warwick, said “With the larger nanoparticles we examined we found that the binding sites for this chemical clamp were the same as the smaller nanoparticles but the binding was much weaker. In the simulations we performed, the protein never desorbed from the smaller nanoparticle, but always fell off or desorbed from the larger one. However in each case, desorption occurred at or after nucleation of calcite.”
The researchers h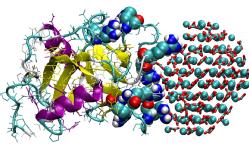 ad therefore uncovered an incredibly elegant process allowing highly efficient recycling of the OC-17 protein. Effectively it acts as a catalyst, clamping on to calcium carbonate particles to kickstart crystal formation and then dropping off when the crystal nucleus is sufficiently large to grow under its own steam. This frees up the OC-17 to promote more yet more crystallisation, facilitating the speedy, literally overnight creation of an egg shell.
ad therefore uncovered an incredibly elegant process allowing highly efficient recycling of the OC-17 protein. Effectively it acts as a catalyst, clamping on to calcium carbonate particles to kickstart crystal formation and then dropping off when the crystal nucleus is sufficiently large to grow under its own steam. This frees up the OC-17 to promote more yet more crystallisation, facilitating the speedy, literally overnight creation of an egg shell.
The researchers believe that this new insight into the elegant and highly efficient methods of promoting and controlling crystallisation in nature will be of great benefit to anyone exploring how to promote and control artificial forms of crystallisation.
Additional quote from the University of Warwick's Professor Mark Rodger added 15th July 2010:
"Does this really prove the chicken came before the egg? Well this actually further underlines that it's a fun but pointless question. This science does however give new insight into an efficient and fast method of crystallisation. It will help in research to devise better synthetic bone and research into how to store/sequester CO2 as limestone."
Note for editors: The paper entitled “Structural Control of Crystal Nuclei by an Eggshell Protein” by Colin L. Freeman , John H. Harding , David Quigley , and Professor P. Mark Rodger, is published in Angewandte Chemie International Edition, DOI: 10.1002/anie.201000679 http://www3.interscience.wiley.com/journal/123506601/abstract 
For further information please contact:
Professor Mark Rodger Department of Chemistry
and Centre for Scientific Computing , University of Warwick
Tel: +44 (0)24 76 523239
p.m.rodger@warwick.ac.uk
Dr David Quigley, Department of Physics and
Centre for Scientific Computing, University of Warwick
44 (0)2476 574580
d.quigley@warwick.ac.uk
or
Peter Dunn, Head of Communications
Communications Office, University House,
University of Warwick, Coventry, CV4 8UW, United Kingdom
email: p.j.dunn@warwick.ac.uk
Tel: +44 (0)24 76 523708 Mobile/Cell: +44 (0)7767 655860
PR61 PJD 9th July 2010
