Start2Quit Results
A randomised controlled trial to encourage smokers to seek help to quit
A personalised invite can encourage more people to quit smoking through Stop Smoking Services
Smoking remains the leading cause of ill health and mortality, at an estimated cost to the NHS of about £6 billion. Stop Smoking Services (SSSs) in England offer intensive advice and support to smokers motivated to quit, and smokers attending have a higher chance of succeeding in a quit attempt than if they attempted to quit alone. However the proportion of smokers using the SSS has been less than 5%, and the number is falling each year. In the Start2quit trial we developed an intervention to persuade and motivate more smokers to seek, or accept, help to quit.
Smokers tend to underestimate their own personal risk. Therefore the intervention consisted of a personal risk information letter that told people about their own risk if they continue to smoke, using information from a screening questionnaire and from medical records. We combined this with an immediate offer of treatment and personal invitation to a no-commitment ‘Come and Try it’ taster session to find out more information about the service and what it involves. These sessions lasted approximately 1 hour were run by advisors, already trained to give smoking cessation advice, and also trained to lead sessions according to a standard protocol.
We assessed whether this intervention would encourage more people to attend the first session of an SSS course compared with a control group who received a standard generic letter advertising the local SSS. We also assessed whether this intervention would increase quit rates, defined as 7-day abstinence at the 6-month follow-up.
Complete data of attendance at the SSS was collected for each participant from SSSs at the end of the 6-month follow-up period, and submitted by SSS staff using NHS monitoring data. Additional data were obtained by telephone interview or postal questionnaire 6-months after the date of randomisation for each participant.
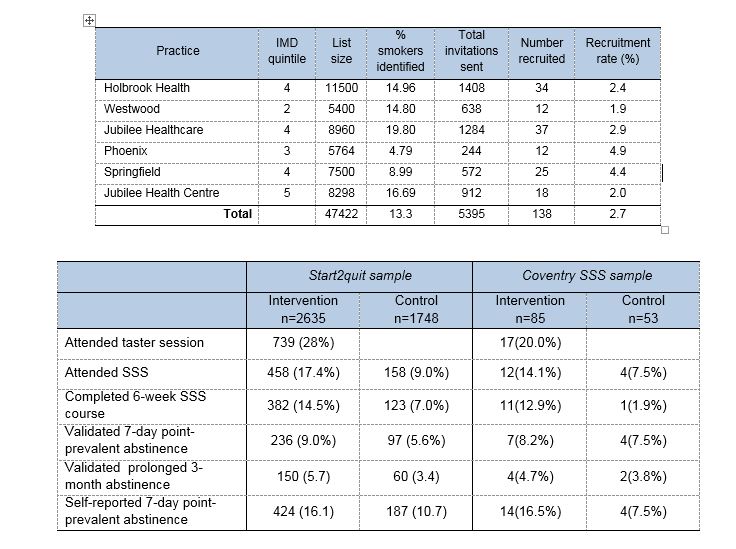
The trial was conducted over 4 years, between January 2011 and December 2014. Eighteen SSSs and 99 practices within the SSS areas were recruited by the Primary Care Research Network (PCRN). All smokers aged 16 and over were identified from medical records in participating practices. After screening by GPs and eliminating duplicate addresses, 112,216 patients were invited to participate. Smokers returning the completed questionnaire and giving consent (4,384 / 4.1%) were allocated at random to the intervention group (2636) or to the control group (1748). A total of 3372 (76.9%) completed the interview or postal questionnaire at the 6-month follow-up (76.7% in the intervention group and 77.3% in the control group).
We attempted to select more practices in areas of high deprivation, determined by the practice postcode converted to an Index of Multiple Deprivation (IMD) score (the Government’s official measure of multiple deprivation at small area level. Consequently 54.6% of practices were located in areas of higher deprivation (i.e. within the two highest quintiles of IMD scores). The practices list sizes ranged from 2,205 to 26,000, and the proportion of smokers identified in each practice ranged from 4.8% to 39.7%.The average age of participants was 49, 51% were male, 51% were living in areas of high deprivation (IMD quintiles 4 and 5), and 32% were living in a household with another smoker. Participants smoked on average 16 cigarettes per day, 68% smoked within 30 minutes of waking. A high proportion (42.5%), were planning to quit sometime in the next 6 months, but not within the next 30 days.
Results
Significantly more people in the intervention group than in the control group attended at least one session of a 6-week SSS course (17.4% vs 9.0%), meaning that receiving a personal letter and invitation to a taster session, rather than receiving a standard letter, more than doubles the odds of attendance. We adjusted the results to account for other factors that might influence attendance, e.g. gender, age, and dependence, and the intervention group still had more than twice the odds of attending. The number of people who completed the 6-week course was also more than doubled in the intervention group (14.5% vs 7.0%).
In addition, significantly more people in the intervention group than in the control group had quit smoking (stopped for at least 7 days and validated by a saliva sample) at the 6-month follow-up (9.0% vs 5.6%), which means that the chances of quitting after receiving this letter and invitation are increased by 61%.
Of the 739 participants in the intervention group who attended a taster session, 338 went on to attend the SSS, and another 120 in the intervention group attended the SSS although they did not attend a taster session. Abstinence rates were highest at 28.7% in those who attended a taster session and also attended the SSS.
Comments from people who attended the tasters sessions suggested that the sessions gave new information and encouraged smokers to look at things in a different way. They were pleased that the advisors did not exert any pressure on the attendees to sign up, and the attendees felt relaxed (‘very good. Without trying to 'preach' or exert undue pressure’), and comments also confirmed that some smokers are not aware of the service and what is offered, e.g. ‘nice to know that such a service exists’
Coventry SSS
The Coventry practices were representative compared of the total sample in terms of deprivation, and showed that we were successful in reaching our target population.
There were large differences in recruitment in SSSs, ranging from 2.3% to 6.7%. Recruitment in Coventry was low overall, but largely due to two practices in particular. This reflected the pattern in other areas, where there were large variations between practices within SSSs. The characteristics of the Coventry sample were very similar to the total in all respects, although the proportion of males was higher at 58%.
Overall SSS attendance also varied between SSSs from 23.1% to as low as 2.1%, and validated 7-day abstinence from 13.4% down to 2.1%. SSS attendance in Coventry SSS was slightly lower than overall attendance, but completion of the course was high, and abstinence rates are comparable with the total. Our criteria for validation are rigorous, and therefore 7-day validated abstinence rates are conservative. Non-validation of 7-day abstinence does not confirm relapse or continued smoking in a participant.
Implications for healthcare
A programme of proactive recruitment can be effective in raising awareness of the SSS, and consequently increasing the service uptake. Personal risk information may prompt more smokers to attempt to quit and the taster sessions offer an opportunity to promote the services in the form of introductory sessions to emphasise their approachability and empathy.
A cost effectiveness analysis suggested that the tailored letter plus the taster session may be more costly during the trial period. However, quitting smoking yields huge health care cost savings and health benefits over the long-term through the reduced risk of smoking-related diseases, such as lung cancer, heart disease, COPD and stroke. The long-term results suggest that over a lifetime horizon, the intervention is more effective and less costly than the generic letter.
Funding:
National Institute for Health Research HTA Programme
Publications:
Gilbert H, Sutton S, Morris R, Petersen I, Galton S, Wu Q, et al. Effectiveness of personalised risk information and taster sessions to increase the uptake of smoking cessation services (Start2quit): a randomised controlled trial. The Lancet , 2017:389(10071): 823–833. http://dx.doi.org/10.1016/S0140-6736(16)32379-0
Gilbert H, Sutton S, Morris R, Petersen I, Wu Q, Parrott S, et al. Start2quit: a randomised clinical controlled trial to evaluate the effectiveness and cost-effectiveness of using personal tailored risk information and taster sessions to increase the uptake of the NHS Stop Smoking Services. Health Technol Assess 2017;21(3) https://dx.doi.org/10.3310/hta21030
For more information contact the PI Dr Hazel Gilbert hazel.gilbert@ucl.ac.uk

