Publications
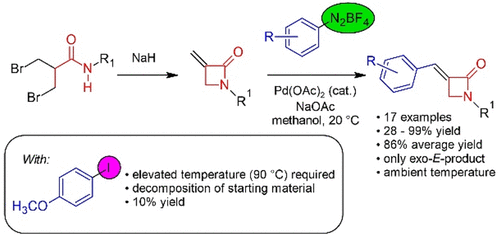
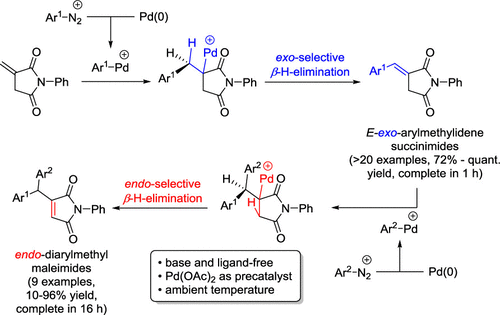
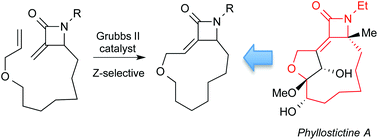
111. Riemer, N.; Riemer, M.; Krueger, M.; Clarkson, G. J.; Shipman, M.; Schmidt, B., Synthesis of Arylidene-β-lactams via Exo-Selective Matsuda-Heck Arylation of Methylene-β-lactams, J. Org. Chem. 2021, 86, 8786−8796. [abstract]
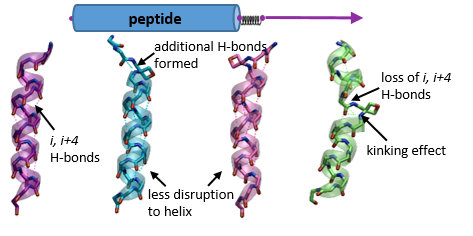
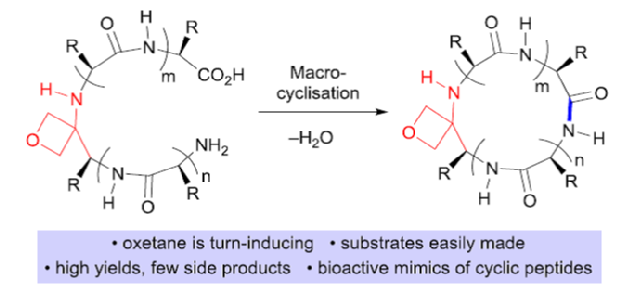
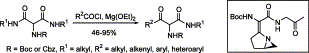
110. Dean, C.; Roesner, S.; Rajkumar, S.; Clarkson, G. J.; Jones, M.; Shipman, M. Synthesis of sp3-rich chemical libraries based upon 1,2-diazetidines, Tetrahedron 2021, 79, 131836. [abstract]
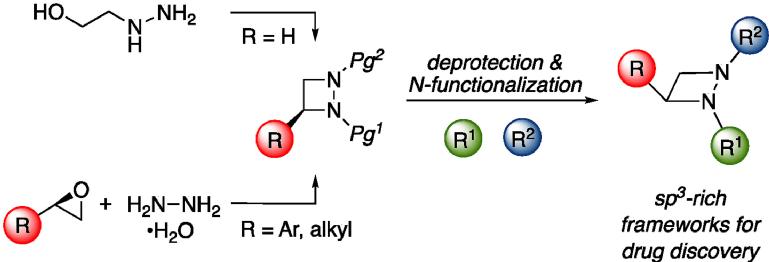
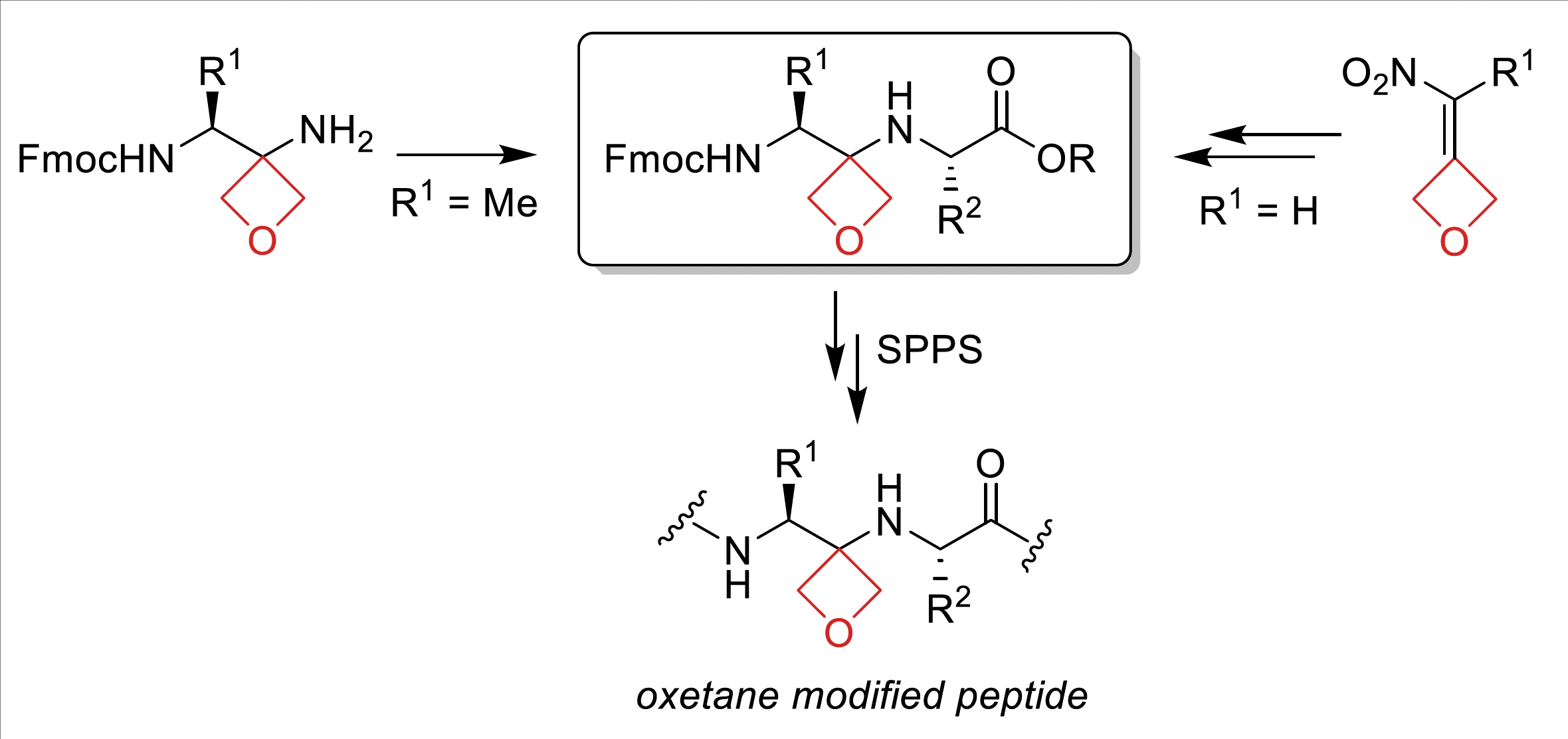

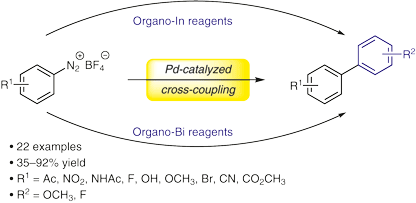
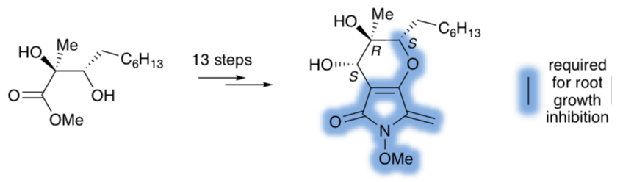
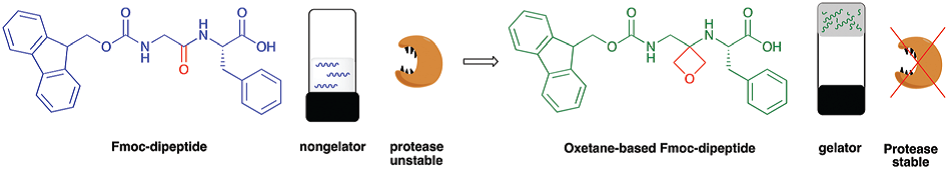
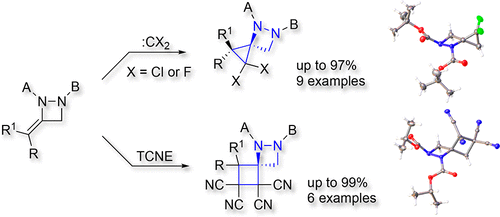
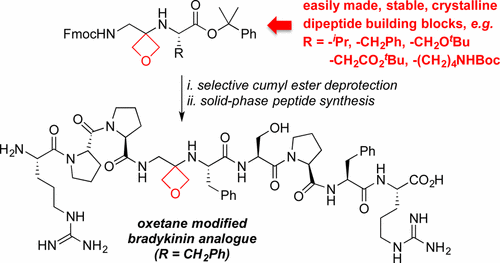
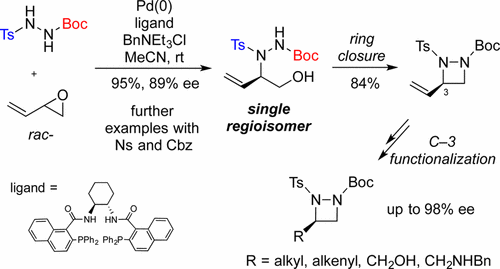
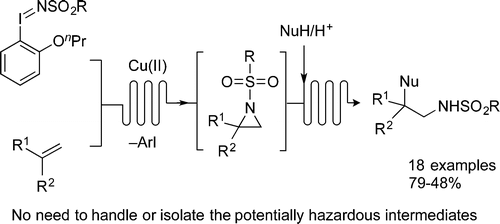
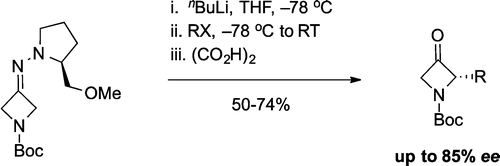
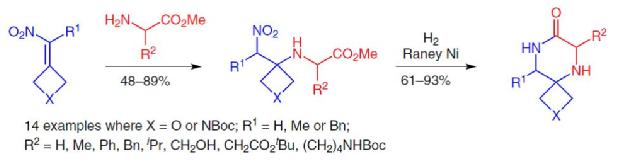
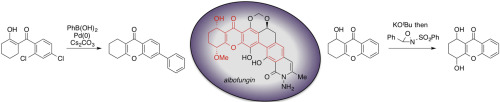
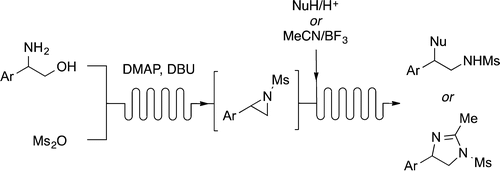
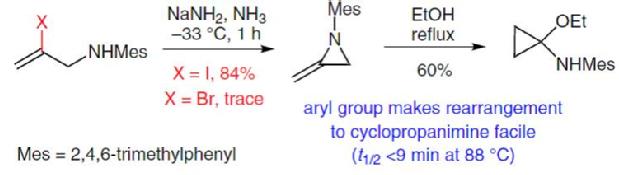
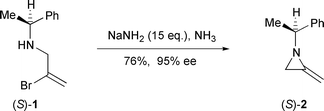
Sodium Amide Induced Cyclization of 2-Iodoprop-2-enylamines: Application to the Synthesis of 1-Aryl-2-methyleneaziridines, Synlett 2015, 26, 1371–1374. [Abstract]
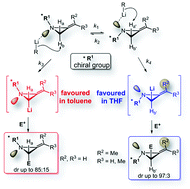
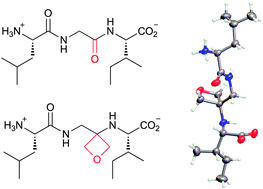
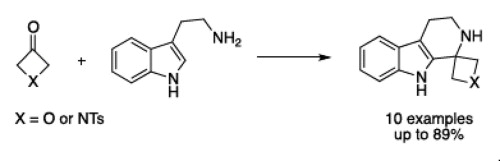
87. Geden, J.V., Beasley, B. O., Clarkson, G. J., Shipman, M., Asymmetric Synthesis of 2-Substituted Oxetan-3-ones via Metallated SAMP/RAMP Hydrazones, J. Org. Chem., 2013, 78, 12243–12250. [Open Access]
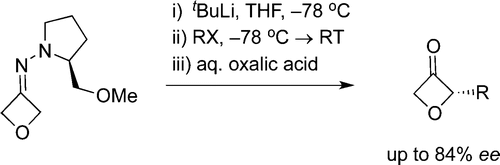
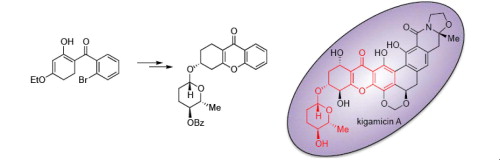
86. Turner, P. A., Samiullah, Whatmore, J. L., Shipman, M., Stereocontrolled synthesis of a D-amicetose functionalised tetrahydroxanthone related to kigamicin A, Tetrahedron Lett., 2013, 54, 6538-6540. [Abstract]
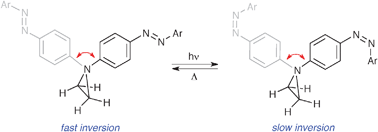
85. Hough, A. J., Prokes, I., Tucker, J. H. R., Shipman, M., Walsh, T.R. Photochemical control of molecular motion associated with pyramidal inversion, Chem. Commun., 2013, 49, 6683-6685. [Abstract]
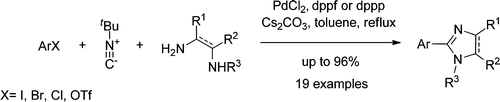
84. Geden, J.V., Pancholi, A.K., Shipman, M., Palladium-Catalyzed Multicomponent Synthesis of 2-Aryl-2-Imidazolines from Aryl Halides and Diamines, J. Org. Chem., 2013, 78, 4158. [Open Access]
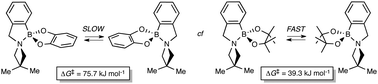
83. Hoang, C. T., Prokes, I., Clarkson, G. J., Rowland, M. J., Tucker, J.H.R., Shipman, M., Walsh, T.R., Study of boron–nitrogen dative bonds using azetidine inversion dynamics, Chem. Commun., 2013, 2509. [Abstract]
82. Aspray, T. J., Jones, E. E., Davies, M. W., Shipman, M., Bending, G. D. Increased hyphal branching and growth of ectomycorrhizal fungus Lactarius rufus by the helper bacterium Paenibacillus sp., Mycorrhiza, 2013, 23, 403-410. [Abstract]

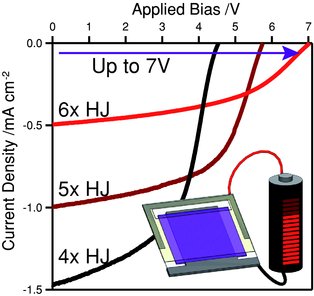
81. Sullivan, P., Schumann, S., Da Campo, R., Howells, T., Duraud, A., Shipman, M., Hatton, R. A., Jones, T. S., Ultra-High Voltage Multijunction Organic Solar Cells for Low-Power Electronic Applications, Advanced Energy Materials, 2013, 3, 239-244. [Abstract]
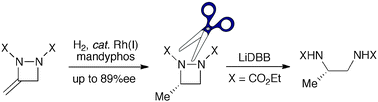
80. Iacobini, G. P., Porter, D. W., Shipman, M. Chemo- and enantioselective Rh-catalysed hydrogenation of 3-methylene-1,2-diazetidines: application to vicinal diamine synthesis, Chem. Commun. 2012, 9852-9854. [Abstract]
79. Griffin, K., Montagne, C., Hoang, C.T., Clarkson, G.J., Shipman, M. Lewis acid promoted intramolecular (3+2) 'cycloadditions' of methyleneaziridines with alkene and alkyne acceptors, Org. Biomol. Chem., 2012, 10, 1032-1039. [Abstract]
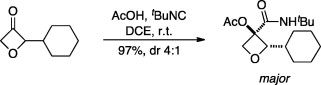
78. Beasley, B. O.; Clarkson, G. J.; Shipman, M. Passerini reactions for the efficient synthesis of 3,3-disubstituted oxetanes, Tetrahedron Lett., 2012, 53, 2951-2953. [Abstract]
77. Sullivan, P., Duraud, A., Hancox, I., Beaumont, N., Mirri, G., Tucker, J. H. R., Hatton, R. A., Shipman, M., Jones, T. S. Halogenated Boron Subphthalocyanines as Light Harvesting Electron Acceptors in Organic Photovoltaics, Advanced Energy Materials, 2011, 1, 352-355. [Abstract]
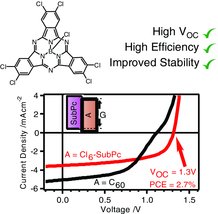
76. Brown, M. J., Clarkson, G. J., Inglis, G. G., Shipman, M. Synthesis and Functionalization of 3-Alkylidene-1,2-diazetidines Using Transition Metal Catalysis, Org. Lett., 2011, 13, 1686-1689. [Abstract]
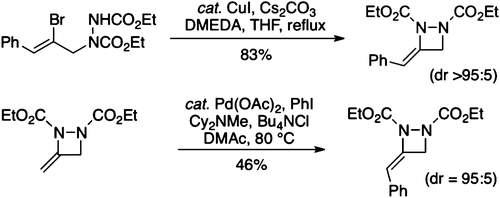
75. Turner, P. A., Griffin, E. M., Whatmore, J. L., Shipman, M. Tetrahydroxanthones by Sequential Pd-Catalyzed C-O and C-C Bond Construction and Use in the Identification of the "Antiausterity" Pharmacophore of the Kigamicins, Org, Lett., 2011, 13, 1056-1059. [Abstract]
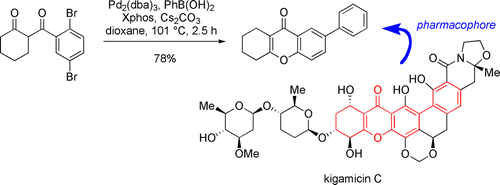
74. Giordano, L., Hoang, C. T., Shipman, M., Tucker, J. H. R., Walsh, T. R. Aziridine Scaffolds for the Detection and Quantification of Hydrogen-Bonding Interactions through Transition-State Stabilization, Angew. Chem. Int. Ed., 2011, 50, 741-744. [Abstract]
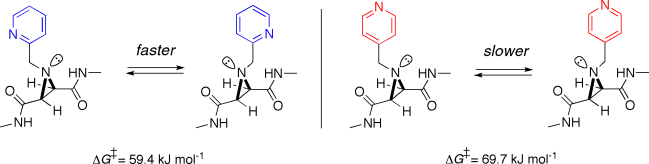
73. Brown, M. J., Clarkson, G. J., Fox, D. J., Inglis, G. G., and Shipman, M., Critical importance of leaving group ‘softness’ in nucleophilic ring closure reactions of ambident anions to 1,2-diazetidines, Tetrahedron Lett., 2010, 51, 382. [Abstract]
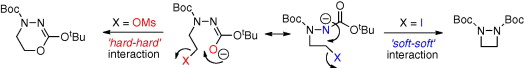
72. Musio, B., Clarkson, G.J., Shipman, M., Florio, S., and Luisi, R., Synthesis of Optically Active Arylaziridines by Regio- and Stereospecific Lithiation of N-Bus-Phenylaziridine, Org. Lett. 2009, 11, 325-328. [Abstract]
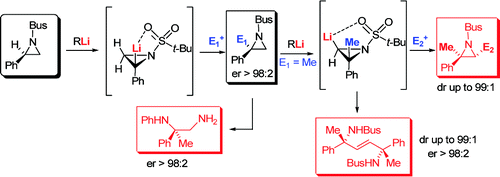
71. Mumford, P.M., Tarver, G.J., and Shipman, M., Four-component reaction for the preparation of a-amino phosphonates from methyleneaziridines, J. Org. Chem., 2009, 74, 3573-3575. [Abstract]
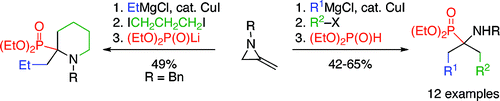
70. Finerty, M. J., Bingham, J. P., Hartley, J. A., Shipman, M., Azinomycin bisepoxides containing rigid aromatic linkers: synthesis, cytotoxicity and DNA interstrand cross-linking activity, Tetrahedron Lett., 2009, 50, 3648-3650. [Abstract]
69. Nalli, S.M., Clarkson, G.J., Franklin, A.S., Bellone, G. and Shipman, M., Iminophosphorane-mediated synthesis of cyclic guanidines: application to the synthesis of a simplified NA22598A1 analogue, Synlett, 2008, 2339-2341. [Abstract]
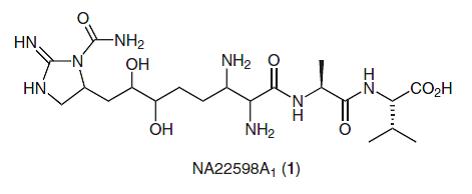
68. Cariou, C.C.A., Clarkson, G.J. and Shipman, M., Rapid Synthesis of 1,3,4,4-Tetrasubstituted β-Lactams from Methyleneaziridines Using a Four-Component Reaction. J. Org. Chem. 2008, 73, 9762-9764. [Abstract]
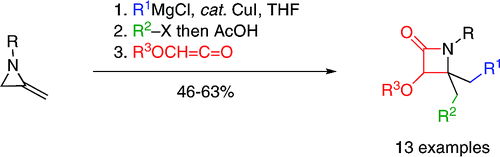
67. Mumford, P.M., Shiers, J.J., Tarver, G.J., Hayes, J.F. and Shipman, M., Synthesis of 1,1-disubstituted tetrahydro-β-carbolines from 2-methyleneaziridines, Tetrahedron Lett. 2008, 49, 3489-3491. [Abstract]
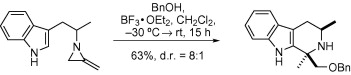
66. Gallienne, E., Muccioli, G.G., Lambert, D.M. and Shipman, M., Microwave-assisted four-component reaction for the synthesis of a monothiohydantoin inhibitor of a fatty acid amide hydrolase, Tetrahedron Lett. 2008, 49, 6495-6497. [Abstract]
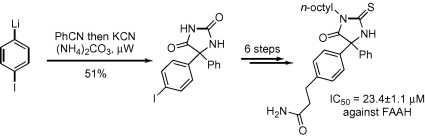
65. Wynne, E.L., Clarkson, G.J. and Shipman, M., Stereocontrolled approach to 1-azabicyclo[4.1.0]heptanes: application to the synthesis of trans-2,6-disubstituted piperidines, Tetrahedron Lett. 2008, 49, 250-252. [Abstract]
![Stereocontrolled approach to 1-azabicyclo[4.1.0]heptanes: application to the synthesis of trans-2,6-disubstituted piperidines](1-s2.0-s0040403907022757-fx1.jpg)
64. Oehlrich, D., Vidot, S.M.E., Davies, M.W., Clarkson, G.J. and Shipman, M., Total synthesis of (±)-luminacin D, Tetrahedron, 2007, 63, 4703-4711. [Abstract]
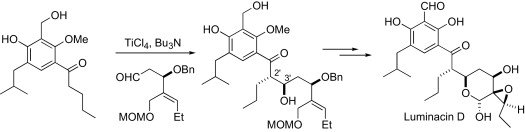
63. Davies, M.W., Clarke, A.J., Clarkson, G.J., Shipman, M. and Tucker, J.H.R., Umbrella motion in aziridines: Use of simple chemical inputs to reversibly control the rate of pyramidal inversion, Chem. Commun., 2007, 5078-5080. [Abstract]
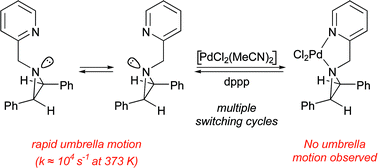
62. Shiers, J.J., Clarkson, G.J., Shipman, M., and Hayes, J.F., Rapid Generation of Molecular Complexity using Hybrid Multi-Component Reactions (MCRs): Application to the Synthesis of α-Amino Nitriles and 1,2-Diamines, Chem. Commun., 2006, 649-651. [Abstract]
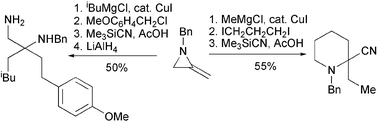
61. Montagne, C., Prévost, N., Shiers, J. J., Prié, G., Rahman, S., Hayes, J. F., and Shipman, M., Generation and Electrophilic Substitution Reactions of 3-Lithio-2-Methyleneaziridines, Tetrahedron, 2006, 8447-8457. [Abstract]
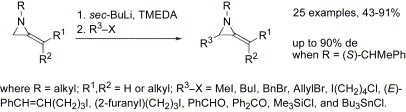
60. Montagne, C., and Shipman, M., Modified Bucherer-Bergs Reaction for the One-Pot Synthesis of 5,5’-Disubstituted Hydantoins from Nitriles and Organometallic Reagents, Synlett, 2006 2203-2206. [Abstract]
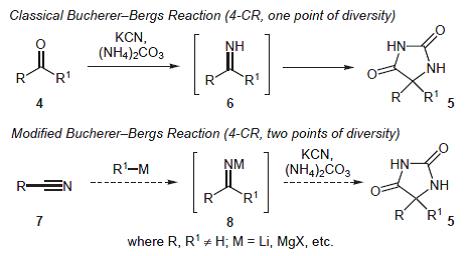
59. Montagne, C., Shiers, J.J. and Shipman, M. Rapid generation of molecular complexity using sequenced multi-component reactions. One-pot synthesis of 5,5'-disubstituted hydantoins from methyleneaziridines, Tetrahedron Lett., 2006, 47, 9207-9209. [Abstract]
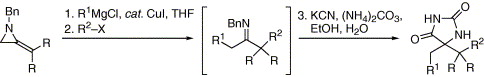
58. Davies, M.W., Shipman, M., Tucker, J.H.R. and Walsh, T.R., Control of Pyramidal Inversion Rates by Redox Switching J. Am. Chem. Soc. 2006, 128, 14260-14261. [Abstract]
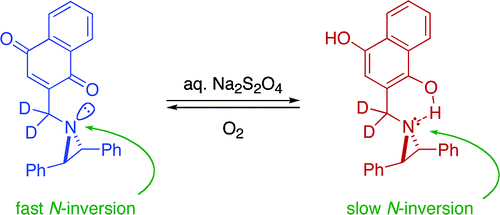
57. Shipman, M.; Montagne, C. UK Pat. Appl. GB0603239.5, 2006.
56. Shipman, M. Methyleneaziridines: Unusual Vehicles for Organic Synthesis, Synlett, 2006, 3205-3217. [Abstract]
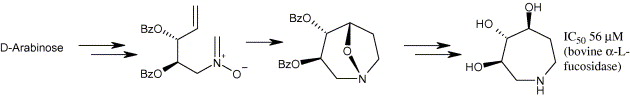
55. Moutel, S., Shipman, M., Martin O.R., Ikeda, K., and Asano, N., Synthesis of a Trihydroxylated Azepane from D-arabinose by way of an Intramolecular Alkene Nitrone Cycloaddition, Tetrahedron: Asymmetry, 2005, 16, 487-491. [Abstract]
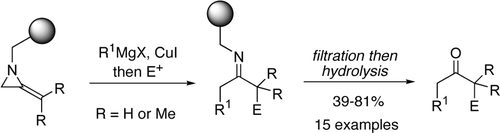
54. Margathe, J.-F., Shipman, M., and Smith, S.C., Solid Phase, Multi-Component Reactions of Methyleneaziridines: Synthesis of 1,3-Disubstituted Propanones, Org. Lett., 2005, 7, 4987-4990. [Abstract]
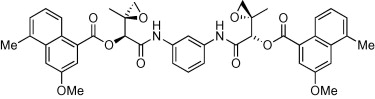
53. LePla, R.C., Landreau, C.A.S., Shipman, M., and Jones, G.D.D., On the Origin of the DNA Sequence Selectivity of the Azinomycins, Org. Biomol. Chem., 2005, 3, 1174-1175. [Abstract]
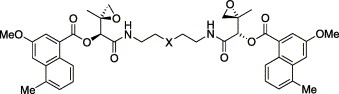
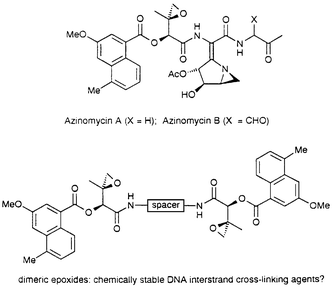
52. LePla, R.C., Landreau, C.A.S., Shipman, M., Hartley, J.A., and Jones, G.D.D., Azinomycin Inspired Bisepoxides: Influence of Linker Structure on in vitro Cytotoxicity and DNA Interstrand Cross-Linking, Bioorg. Med. Chem. Lett., 2005, 15, 2861-2864. [Abstract]
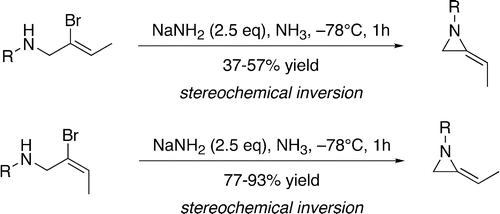
51. Shiers, J.J., Shipman, M., Hayes, J.F., and Slawin, A.M.Z., and Twin, H., Rare Example of Nucleophilic Substitution at Vinylic Carbon with Inversion: Mechanism of Methyleneaziridine Formation by Sodium Amide Induced Ring Closure Revisited, J. Am. Chem. Soc., 2004, 126, 6868-6869. [Abstract]
50. Paumier, D., Garcia, M., Shipman M., and Muir, J.C., Rapid Assembly of the 1-Azabicyclo[3.1.0]hexane Skeleton of Ficellomycin, Synlett, 2004, 2212-2214. [Abstract]
49. Prié, G., Prévost, N., Twin, H., Fernandes, S.A., Hayes, J. F., and Shipman M., A Lewis Acid Catalyzed Intramolecular [4+3] Route to Polycyclic Systems Containing a Seven Membered Ring, Angew. Chem. Int. Ed., 2004, 43, 6517-6519. [Abstract]
![A Lewis Acid Catalyzed Intramolecular [4+3] Cycloaddition Route to Polycyclic Systems That Contain a Seven-Membered Ring](mcontent_2.gif)
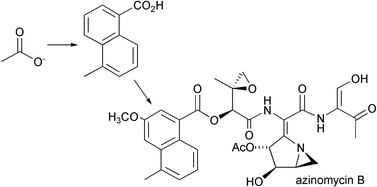
48. Corre, C., Landreau, C. A. S., Shipman, M., and Lowden, P. A. S., Biosynthetic Studies on the Azinomycins: The Pathway to the Naphthoate Fragment, Chem. Commun., 2004, 2600-2601. [Abstract]
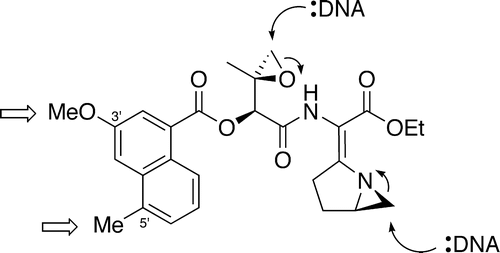
47. Landreau, C. A. S., LePla, R. C., Shipman, M., Slawin, A. M. Z., and Hartley, J. A., Delineating Non-Covalent Interactions Between the Azinomycins and Double Stranded DNA: Importance of the Naphthalene Substitution Pattern on Interstrand Cross-Linking Efficiency, Org. Lett., 2004, 6, 3505-3507. [Abstract]
46. Davies, M. W., Maskell, L., Shipman, M., Slawin, A. M. Z., Vidot, S. M. E., and Whatmore, J. L., Studies Towards the Synthesis of Luminacin D: Assembly of Simplified Analogues Devoid of the Epoxide Displaying Anti-Angiogenic Activity, Org. Lett., 2004, 6, 3909-3912. [Abstract]
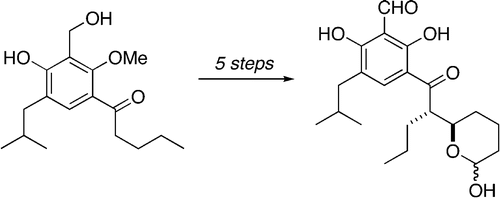
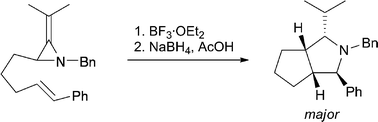
45. Hayes, J.F., Prévost, N., I. Prokes, Shipman, M., Slawin, A.M.Z., and Twin, H., Aziridinyl Anions from a Chiral, Nonracemic 2-Isopropylideneaziridine: Surprisingly Diastereoselective Alkylation Reactions, Chem. Commun., 2003, 1344-1345. [Abstract]
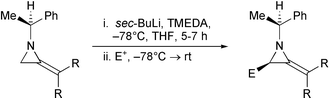
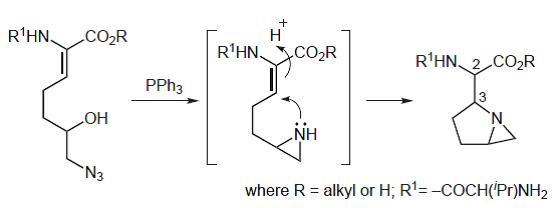
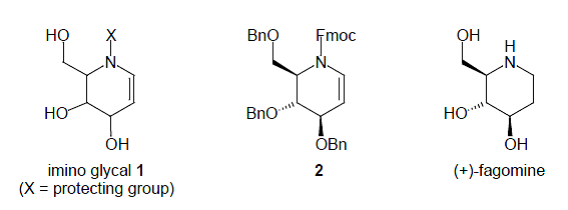
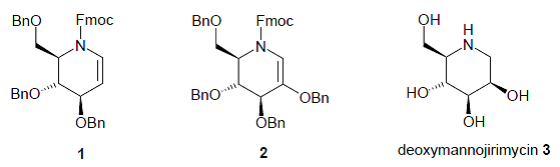
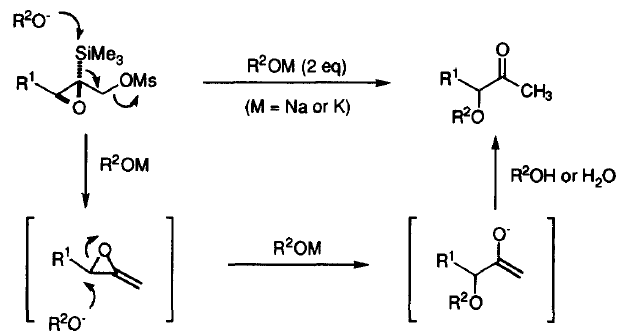
44. Dransfield, P.J., Gore, P.M., I. Prokes, Shipman, M., and Slawin, A.M.Z., Preparation and Reactivity of Imino Glycals: Stereocontrolled Divergent Approach to Imino Sugars, Org. Biomol. Chem., 2003, 2723-2733. [Abstract]
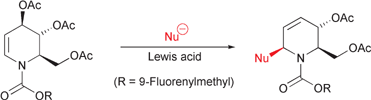
43. Hayes, J.F., Shipman, M., and Twin, H., Multi-Component Reactions Involving 2-Methyleneaziridines: Rapid Synthesis of 1,3-Disubstituted Propanones, J. Org. Chem., 2002, 67, 935-942. [Abstract]
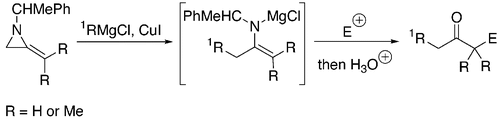
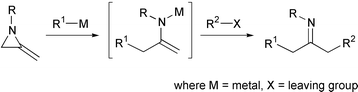
42. Dransfield, P.J., Gore, P.M., Shipman, M., and Slawin, A.M.Z., Divergent Approach to Imino Sugar C-Glycosides using Imino Glycals: Application to the Stereocontrolled Synthesis of (+)-Deoxoprosophylline, Chem Commun., 2002, 150-151. [Abstract]

41. Hayes, J.F., Shipman, M., Slawin, A.M.Z., and Twin, H., Multi-Component Reactions of 2-Isopropylideneaziridines: Application to the Synthesis of Enantiopure Neopentylamines, Heterocycles, 2002, 58, 243-250. [Abstract]
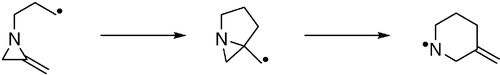
40. Prévost, N., and Shipman, M., Synthesis of Substituted Piperidines, Decahydroquinolines and Octahydroindolizines by Radical Rearrangement Reactions of 2-Alkylideneaziridines, Tetrahedron, 2002, 58, 7165-7175. [Abstract]
![]()
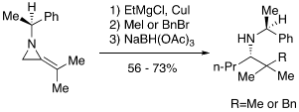
38. Hayes, J.F., Shipman, M., and Twin, H., Asymmetric Synthesis of 2-Substituted Piperidines using a Multi-Component Coupling Reaction: Rapid Assembly of (S)-(+)-Coniine from (S)-1-Phenylethyl-2-methyleneaziridine, Chem. Commun., 2001, 1784-1785. [Abstract]
36. Désiré, J., Dransfield, P.J., Gore, P.M., and Shipman, M., Chemistry of Imino Glycals: Preparation and Application to the Synthesis of (+)-Fagomine, Synlett, 2001, 1329-1331. [Abstract]
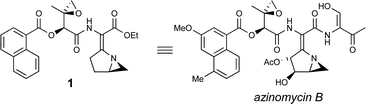
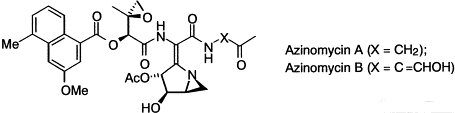
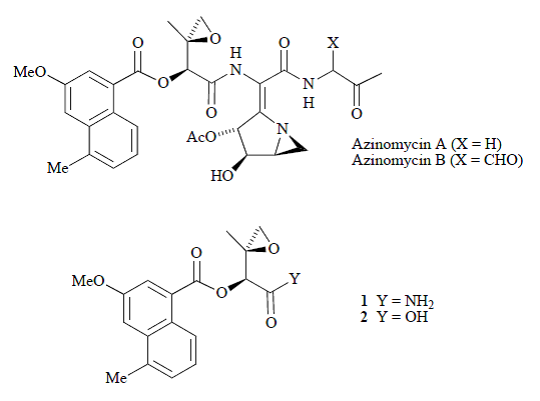
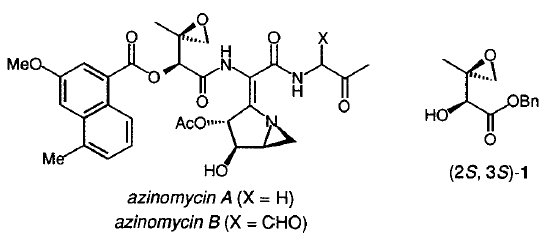
34. Hodgkinson, T.J., and Shipman, M., Chemical Synthesis & Mode of Action of the Azinomycins, Tetrahedron, 2001, 57, 4467-4488. [Abstract]
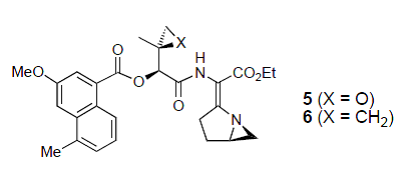
32. Hodgkinson, T.J., Kelland, L.R., Shipman, M., and Suzenet, F., Chemical Synthesis and Cytotoxicity of Some Azinomycin Analogues Devoid of the 1-Azabicyclo[3.1.0]hexane Subunit, Bioorg. Med. Chem. Lett., 2000, 10, 239-241. [Abstract]
![Chemical synthesis and cytotoxicity of some azinomycin analogues devoid of the 1-azabicyclo[3.1.0]hexane subunit](1-s2.0-s0960894x99006630-gr1_lrg.gif)
30. Clapham, G. and Shipman, M., Selective Lewis Acid Complexation of 2-Hydroxyethyl Esters Using Competitive Diels-Alder Reactions as a Mechanistic Probe, Tetrahedron, 2000, 56, 1127-1134. [Abstract]
28. Hartley, J.A., Kelland, L.R., Hazrati, A., Khanim, R.; Shipman, M., Suzenet F., and Walker, L.F., A Synthetic Azinomycin Analogue With Demonstrated DNA Cross-Linking Activity: New Insights into the Mechanism of Action of this Class of Anti-tumour Agent, Angew. Chem. Int. Ed., 2000, 39, 3467-3470. [Abstract]

26. Moutel, S., and Shipman, M., Synthesis of (+)-Nojirimycin from 2,3,4,6-Tetra-O-benzyl-D-glucopyranose, J. Chem. Soc., Perkin Trans. 1, 1999, 1403-1406. [Abstract]
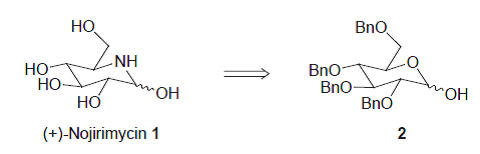
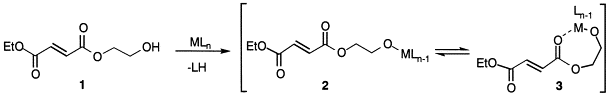
25. Clapham, G. and Shipman, M., Selective Complexation of 2-Hydroxyethyl Esters Using Lewis Acids, Tetrahedron Lett., 1999, 40, 5639-5642. [Abstract]
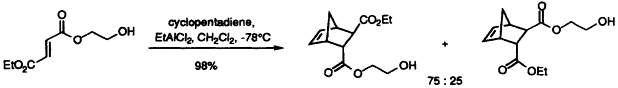
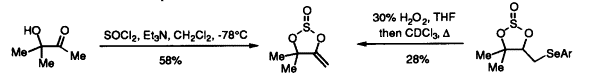
24. Clemens, I.R., Shipman, M., and Thorpe, H.R., Synthesis of 1,3,2-Dioxathiolane-4-Methylene-2-Oxides: Potential Allene Oxide Equivalents, Tetrahedron, 1999, 55, 10845-10850. [Abstract]
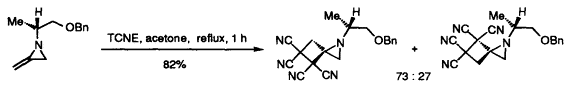
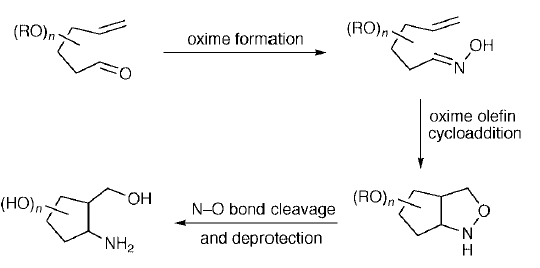
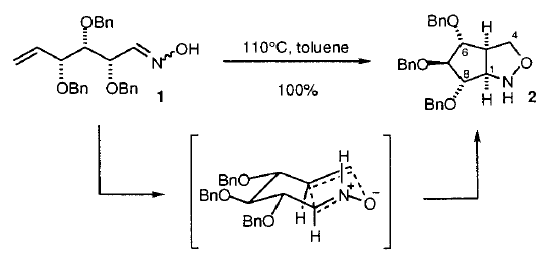
22. Dransfield, P.J., Moutel, S., Shipman, M., and Sik, V., Stereocontrolled Synthesis of Polyhydroxylated Hexahydro-1H-Cyclopent[c]isoxazoles by Intramolecular Oxime Olefin Cycloadditions: An Approach to Aminocyclopentitols, J. Chem. Soc., Perkin Trans. 1, 1999, 3349-3356. [Abstract]
20. Hodgkinson, T.J., Kelland, L.R., Shipman, M., and Vile, J., Synthesis and Reactivity of Some Chiral, Nonracemic 1-Azabicyclo[4.1.0]heptanes Related to the Azinomycins, Tetrahedron, 1998, 54, 6029-6034. [Abstract]
![Synthesis and reactivity of some chiral, nonracemic 1-azabicyclo[4.1.0]heptanes related to the azinomycins](1-s2.0-s004040209800283x-gr1.gif)
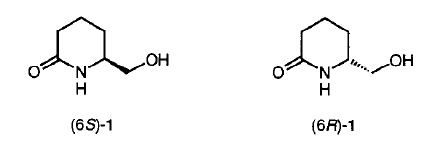
18. Hodgkinson, T.J. and Shipman, M., Practical Asymmetric Synthesis of Both Enantiomers of (6-Hydroxymethyl)-2-piperidinone, Synthesis, 1998, 1141-1144. [Abstract]
16. Clemens, I.R., Shipman, M., and Thorpe, H.R., Synthesis of Chiral α-Alkoxyketones via Allene Oxides, Tetrahedron Lett., 1997, 38, 897-900. [Abstract]

15. Ince, J., Shipman, M., and Ennis, D.S., Novel Ring Opening Reactions of Methyleneaziridines, Tetrahedron Lett., 1997, 38, 5887-5890. [Abstract]
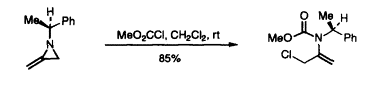
14. Binger, P., Corlay, H., Motherwell, W.B., Pennell, A.M.K., Shipman, M., Stepp, M., Slawin, A.M.Z., and Williams, D.J., Stereochemical Aspects of Intramolecular Palladium Catalysed [3+2] Cycloadditions of Methylenecyclopropanes, Tetrahedron, 1996, 52, 4883-4902. [Abstract]
![Stereochemical aspects of intramolecular palladium catalysed [3+2] cycloadditions of methylenecyclopropanes](1-s2.0-0040402096001603-gr1.gif)
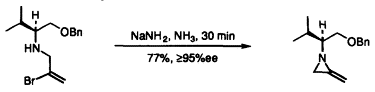
12. Bryant, H.J., Dardonville, C.Y., Hodgkinson T.J., Shipman, M., and Slawin, A.M.Z., Asymmetric Synthesis of the Epoxide Portion of the Azinomycins, Synlett, 1996, 973-974. [Abstract]
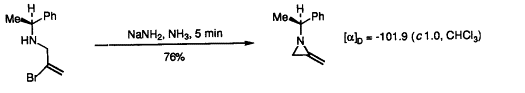
10. Corlay, H., Lewis, R.T., Motherwell, W.B., and Shipman, M., Intramolecular Palladium Catalysed [3+2] Cycloadditions of Methylenecyclopropanes with Acetylenic Acceptors, Tetrahedron, 1995, 51, 3303-3318. [Abstract]
![Intramolecular palladium catalysed [3+2] cycloadditions of methylenecyclopropanes with acetylenic acceptors](1-s2.0-004040209500052a-gr1.gif)
![An improved preparation of diphenylmethylenecyclopropanes and their use in intramolecular palladium catalysed [3+2] cycloadditions](1-s2.0-0040402095000519-gr1.gif)
8. Clemens, I.R., Shipman, M., and Thorpe, H.R., An Improved Procedure for the Generation and In Situ Trapping of Allene Oxides with Alcohols, Synlett, 1995, 1065-1066. [Abstract]
6. Shipman, M., Phenyl(tribromomethyl)mercury, Encyclopedia of Reagents for Organic Synthesis, L.A. Paquette (Ed.), Wiley, 1995, 6, 4090. [Abstract]

5. Shipman, M., Bis(trimethylsilyl)mercury, Encyclopedia of Reagents for Organic Synthesis, L.A. Paquette (Ed.), Wiley, 1995, 1, 598. [Abstract]

3. Motherwell, W.B. and Shipman, M., Intramolecular Cycloadditions of Methylenecyclopropanes: a Novel Chelation Effect, Tetrahedron Lett., 1991, 32, 1103-1107. [Abstract]
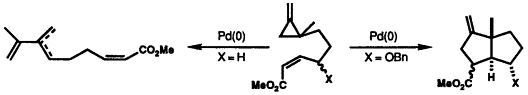
2. Bapuji, S.A., Motherwell, W.B., and Shipman, M., Regiospecific Intramolecular [2π+2σ] Cycloadditions of Methylenecyclopropanes, Tetrahedron Lett., 1989, 30, 7107-7110. [Abstract]
![Regiospecific intramolecular [2π+2σ] cycloadditions of methylene cyclopropanes](1-s2.0-s0040403901934367-gr1.gif)
1. Lewis, R.T., Motherwell, W.B., and Shipman, M., Observations on the Intramolecular Palladium (0) Catalysed [3+2] Cycloaddition of Diphenylmethylenecyclopropanes, J. Chem. Soc., Chem. Commun., 1988, 948-950. [Abstract]
![Observations on the intramolecular palladium(0) catalysed [3 + 2] cycloaddition of diphenylmethylenecyclopropanes](observations_on_the_intramolecular_palladium0_catalysed_3__2_cycloaddition_of_diphenylmethylenecyclopropanes.png)