The Solution Phase
Project Overview
Our vision has been to transition our gas-phase expertise to study biological processes in solution, which ultimately provides a much better method for replicating biological environments. To this end, we have developed a transient absorption spectroscopy (TAS) setup in our laboratory allowing us to study photochemical processes in a wide range of solutions. The results of some exemplar studies are posted below. The setup is being applied to the study of:
- Complementary condensed-phase photodissociation studies of biomolecules (see The Gas Phase work for more information)
- Photochemistry of sunscreen molecules including both artificial and nature derived molecules
- Photoinitiated transition metal complexes for application to anti-cancer drugs
- Porphyrin and polymer-based photochemistry
Current Experimental Setup
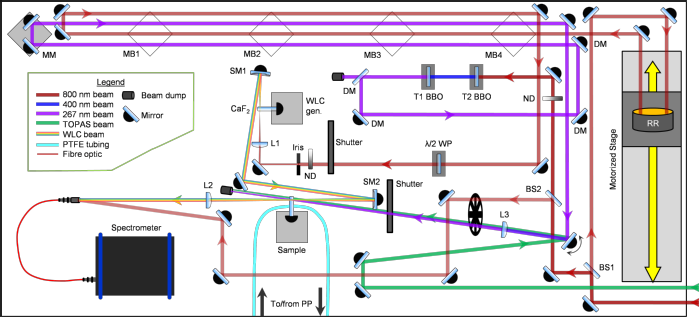
The current experimental arrangement for the transient absorption studies is displayed above. Femtosecond laser pulses are produced by a commercial Ti-Sapphire regenerative amplified laser system (Spectra-Physics, Spitfire XP), which outputs 40 fs, 3 mJ pulses centred at 800 nm with a 1 kHz repetition rate. The beam is split into three parts: one beam for a harmonic pump generation source, one for an optical parametric amplifier (OPA) (Light Conversion, TOPAS-C (UV-VIS)) pump source, and one for white light continuum (WLC) probe generation. The OPA provides pump pulses with tuneable wavelengths in the range 240 – 1160 nm and typical powers of 8 – 90 μJ / pulse, while harmonic generation provides either 400 or 267 nm, >100 μJ pump pulses. The WLC probe, spanning a range of 325 – 750 nm, is generated by focusing a weak 800 nm beam into a vertically translated 1 mm thick calcium fluoride (CaF2) window; this path is shown below.
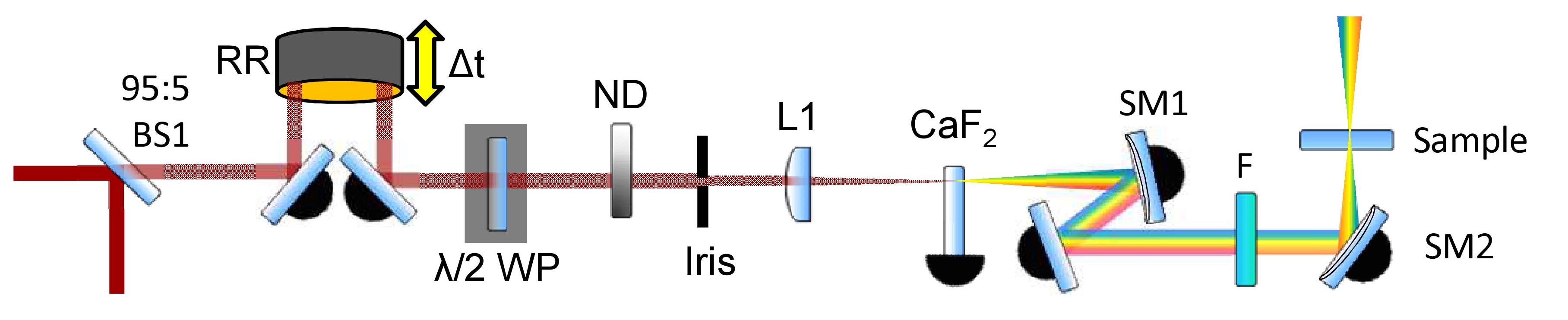
A summarized white-light continuum probe generation beam path. BS – beamsplitter, RR – retroreflector, λ/2 WP – half-waveplate, ND – neutral density filter, L – lens, SM – spherical mirror, F – filter

A hollow gold retroreflector mounted on a motorized translation in the pre-WLC path creates a variable pump-probe time delay ranging from femtoseconds (fs) to picoseconds (ps). Use of optics on flip mounts and prepositioned magnetic bases gives extra delay lines for select longer, nanosecond (ns) pump-probe time delays.
Specifications:
- UV pump excitation: 267/400 nm HG or tuneable UV/Vis 240 – 1160 nm output from an OPA
- White light continuum (WLC) probe: 325-750 nm bandwidth
- Sample delivery by flow-through cell (plus inert/controlled atmosphere) or thin-film liquid gravity jet.
Example Studies
1. Sunscreen Photochemistry
Oxybenzone
Oxybenzone is a UV chromophore which is a common constituent of many commercial sunscreens. As is the case with many of the ingredients of sunscreens, oxybenzone is used as it displays broadband absorption across the UV regions, but, the energy dissipation mechanism is not well understood. After insightful Ab initio electronic structure calculations by Karsili et al. [1] which indicated that oxybenzone may exhibit an enol → keto tautomerisation in order to relax to its ground state after UV irradiation, we combined transient electronic absorption spectroscopy (TEAS, Figure 1.1 left) and a complementary technique, transient vibrational absorption spectroscopy (TVAS, Figure 1.1 right) in collaboration with the Ashfold group in Bristol to try to understand this process experimentally.
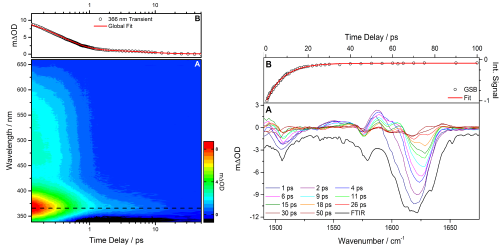
Figure 1.1. Left: (A) Transient electronic absorption of oxybenzone in cyclohexane after 325 nm irradiation. A decay of the intense peak centred at 366 nm is due to the relaxation processes of oxybenzone which is fitted with two exponential function, see (B), to determine the lifetimes of the processes. Right: (A) Transient vibrational absorption spectroscopy is used in a complementary fashion and the integrated area of the ~1630 cm-1 ground state bleach (GSB) is calculated and tracked over time, which when fitted with a single exponential function, see (B), agrees with the long time process determined from TEAS.
As described in our recent work [2], we shed light on the energy dissipation mechanism of oxybenzone, consistent with a ultrafast enol-keto tautomerisation followed by C-C bond rotation and subsequent internal conversion relaxation processes with a small proportion of photoproducts forming.
References:
[1] Karsili, T. N. V. et al., J. Phys. Chem. A, 118 (2014) 11999.
[2] Baker, L. A. et al., J. Phys. Chem. Lett., 6 (2015) 1363.
2. Transition Metal Complexes
Along with the Sadler group we are exploring the ways in which transition metal complexes may be activated using light to generate new drugs to treat diseases such as cancer. Traditional chemotherapeutic agents not only kill cancerous cells but healthy ones too, leading to severe side effects. By starting with a nontoxic precursor molecule that can be activated in a specific area of the body through illumination, we potentially have a highly specific, non-invasive treatment.
An example study examines the mechanism of photoactivation of cis-[Ru(bipyridine)2(nicotinamide)2]2+ (1 - see diagram below) [1] a photoactive species designed to display high cytotoxicity following irradiation. In Ru(II) complexes of this type, efficient population of a dissociative triplet metal-centred (3MC) state is key to generating high quantum yields of a penta-coordinate intermediate (PCI) species [2], which in turn may form the target species: a mono-aqua photoproduct [Ru(bipyridine)2(nicotinamide)(H2O)]2+ (2). Following irradiation of 1, a thorough kinetic picture is derived from ultrafast UV/Vis transient absorption spectroscopy measurements provides both timescales and quantum yields for the key processes involved. We show that photoactivation of 1 to 2 occurs with a quantum yield ≥0.36; all within a timeframe of 400 ps. Characterization of the excited states and the initial steps involved in this photoactivation provides key information for furthering the design of more efficacious photochemotherapy drugs of this type.
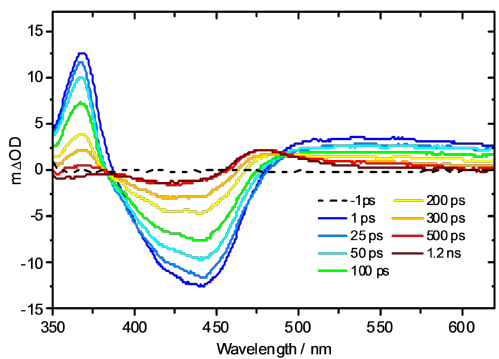
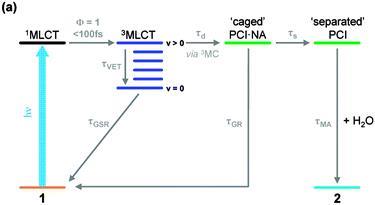
References:
[1] S. E. Greenough et al., Phys. Chem. Chem. Phys., 16 (2014) 19141
[2] P. S. Wagenknecht and P. C. Ford, Coord. Chem. Rev., 255 (2011) 591
3. Porphyirin Photochemistry
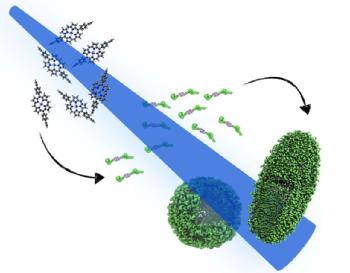
In collaboration with the O’Reilly group, we are investigating an alternative approach for creating biomimetic multi-chromophoric arrays. First, we design and synthesise porphyrin containing polymer systems that will self-assemble upon introduction to solution. Then, a bottom-up approach is applied to the spectroscopic investigation of the ecited state dynamics of these materials. We start by exploring the photodynamics in the single porphyrin molecules before moving on to the polymer functionalised version [1-2]. Finally we investigate the large assembled macro-structures to see how the electronic energy levels of the system are affected following the drastic increases in molecular complexity.
References:
[1] W.D. Quan et al., Chem. Comm., 52 (2016) 1938
[2] W.D. Quan et al., Chem. Phys. Lett., 777 (2021) 138736
