Wills Group Publications
Research publications (click for - reviews/books and posters) Papers published since 2005 are also on Warwick WRAP (Warwick Research Archive Portal).
174) Single-benzene-based clickable fluorophores for in vitro and in vivo bioimaging. Raja Mohanrao, Clyde S. Pinto, Andrejus Suchenko, Guy J. Clarkson, Martin Wills, Stefan Roesner, Michael Shipman, Mohan K. Balasubramanian, ChemRxiv, deposited 26 February 2024.
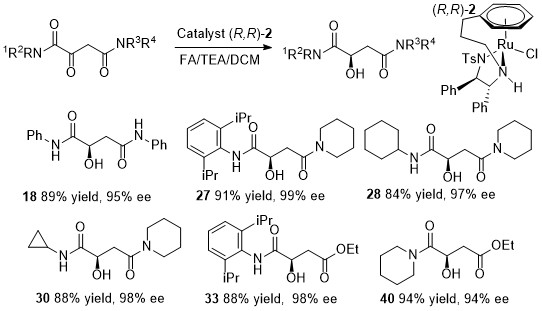
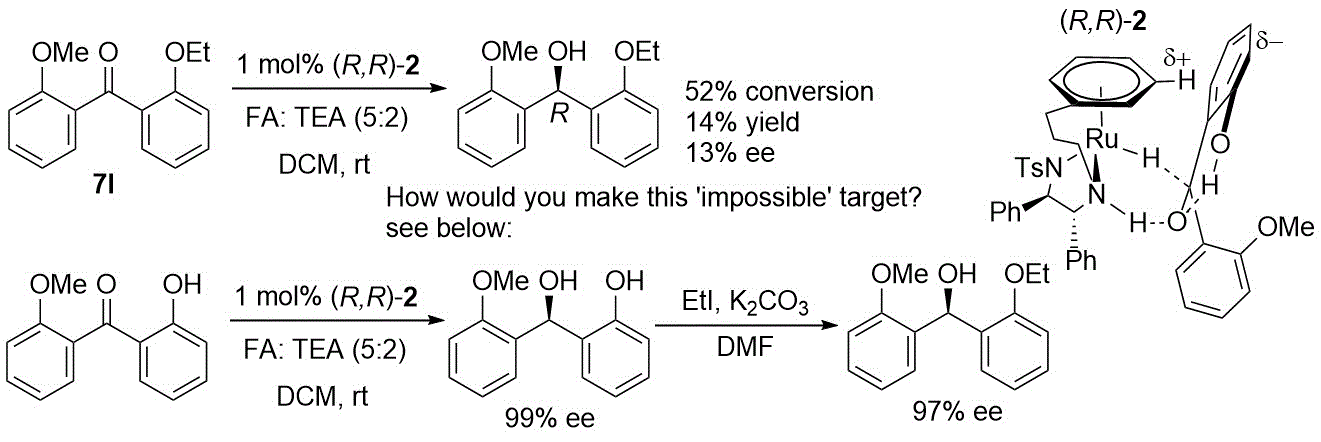
173) Regio- and Enantioselective Asymmetric Transfer Hydrogenation of One Carbonyl Group in a Diketone through Steric Hindrance, Noha Khamis, Ye Zheng, Marianna N. Diamantakis, Guy J. Clarkson, Jie Liu, and Martin Wills, J. Org. Chem. 2024, 89, 2759–2763. Published online 3rd February 2024.
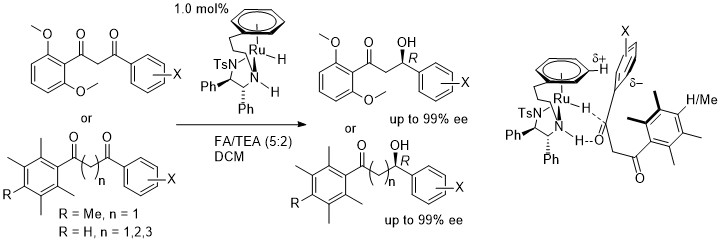
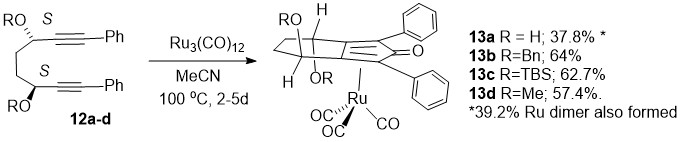
172) Increasing the versatility of the biphenyl-fused-dioxacyclodecyne class of strained alkynes. Sam Forshaw, Jeremy S. Parker, William T. Scott, Richard C. Knighton, Neelam Tiwari, Samson M. Oladeji, Andrew C. Stevens, Yean Ming Chew, Jami Reber, Guy J. Clarkson, Mohan K. Balasubramanian and Martin Wills, Org. Biomol. Chem. 2024, 22, 590-605. DOI: 10.1039/D3OB01712E. Accepted Manuscript 8/12/2023.
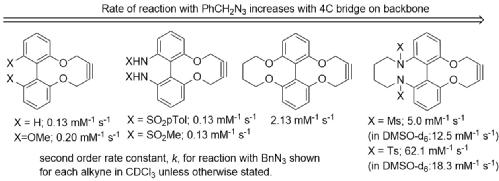
171) Chemoselective derivatisation and ultrahigh resolution mass spectrometry for the determination of hydroxyl functional groups within complex bio-oils. Diana Catalina Palacio Lozano, Hugh E. Jones, Mark P. Barrow and Martin Wills, RSC Advances, 2023, 13, 17727-17741.
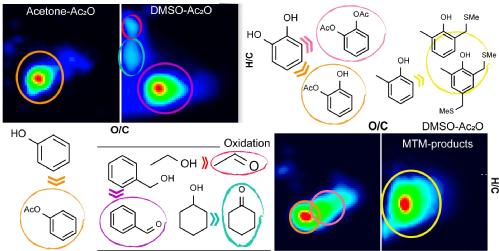
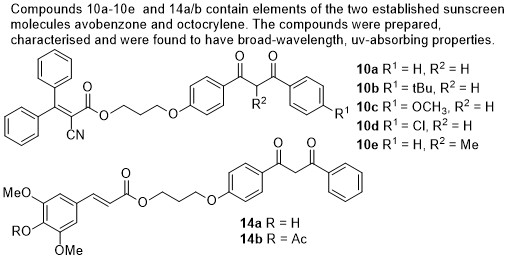
170) Synthesis and Characterisation of Novel Composite Sunscreens Containing both Avobenzone and Octocrylene Motifs, Adam M. Cowden, Abigail L. Whittock, Emily L. Holt, Vasilios G. Stavros and Martin Wills, RSC Advances, 2023, 13, 17017 – 17027.
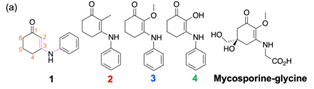
169) Examining the substituent effect on mycosporine-inspired ultraviolet filters. Abigail L. Whittock, Adam M. Cowden, Martin Wills and Vasilios G. Stavros, Phys. Chem. Chem. Phys. 2023, 25,7401-7406. Published online, 06 Feb 2023. A collaborative project led by Prof Vas Stavros.
A series of simplified mycosporine-glycine analogues were prepared through a short sequence. The compounds demonstrated photoprotective properties.
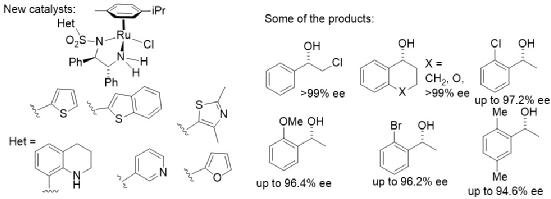
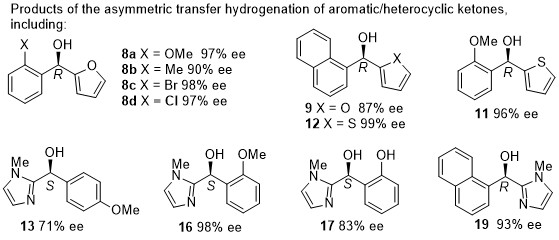
168) Heterocycle-containing Noyori-Ikariya Catalysts for Asymmetric Transfer Hydrogenation of Ketones, Noha Khamis, Guy J. Clarkson and Martin Wills, Dalton Transactions, 2022, 51, 13462 - 13469, DOI: 10.1039/D2DT02411J, published online 19/8/2022.
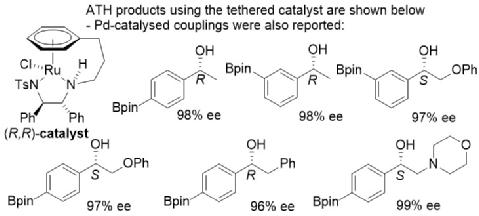
167) Asymmetric transfer hydrogenation of boronic acid pinacol ester (Bpin)-containing acetophenones. Ye Zheng and Martin Wills, Org. Biomol. Chem. 2022, 20, 3742 - 3746. Accepted 12th April 2022. DOI: 10.1039/d2ob00569g.
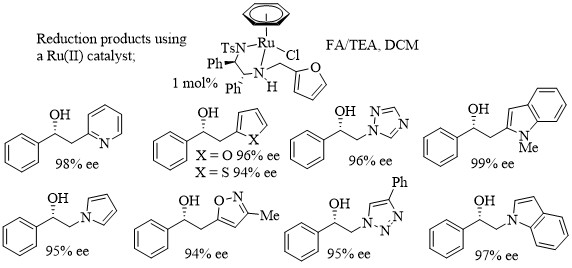
166) Asymmetric transfer hydrogenation of heterocycle-containing acetophenone derivatives using N-functionalised [(benzene)Ru(II)(TsDPEN)] complexes, Jonathan Barrios-Rivera, YingjianXu, Guy J.Clarkson and Martin Wills, Tetrahedron 2022, 103, 132562. Available online 19 November 2021, Article 132562. Sharelink to free access until 14th January 2021.
165) Asymmetric Transfer Hydrogenation of α-Keto Amides; Highly Enantioselective Formation of Malic Acid Diamides and α-Hydroxyamides, Shweta K. Gediya, Vijyesh K. Vyas, Guy J. Clarkson, and Martin Wills, Org. Lett. 2021, 23, 7803-7807. Published online 29/9/2021.
164) 'Asymmetric transfer hydrogenation of aryl heteroaryl ketones using Noyori-Ikariya catalysts', Ye Zheng, Jaime Martinez-Acosta, Mohammed Khimji, Luiz Claudio Barbosa, Guy J. Clarkson and Martin Wills ChemCatChem. 2021, 13, 4384-4391. Accepted article online 23rd July 2021; Article share to a readably copy here .
Review 36) Book Chapter 'Tethered Ruthenium Catalysts in Asymmetric Transfer Hydrogenation', Chapter 7 in Asymmetric Hydrogenation and Transfer Hydrogenation, 2021, Wiley-VCH, Editors: Phannarath Phansavath, Virginie Ratovelomanana-Vidal. ISBN: ISBN: 978-3-527-34610-3.
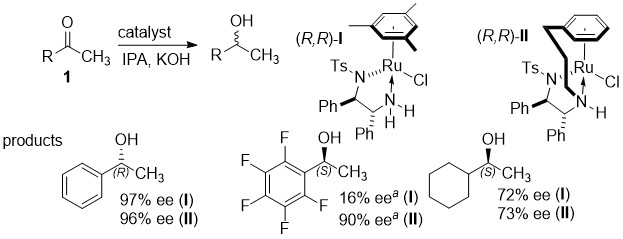
163) Enantioselectivity in the Noyori-Ikariya Asymmetric Transfer Hydrogenation of Ketones. Pavel A. Dub*, Nikolay V. Tkachenko, Vijyesh K. Vyas, Martin Wills, Justin S. Smith, and Sergei Tretiak. Organometallics, 2021 40, 1402–1410, ASAP. Publication Date (Web):April 27, 2021. This is a computational study to explain the differences in selectivity in the reactions below:
162) Exploring the Blueprint of Photoprotection in Mycosporine-like Amino Acids, Abigail L. Whittock, Nazia Auckloo, Adam M. Cowden, Matthew A. P. Turner, Jack M. Woolley, Martin Wills, Christophe Corre, and Vasilios G. Stavros, The Journal of Physical Chemistry Letters, 2021, 12, 3641–3646. ASAP. Publication Date (Web):April 7, 2021.

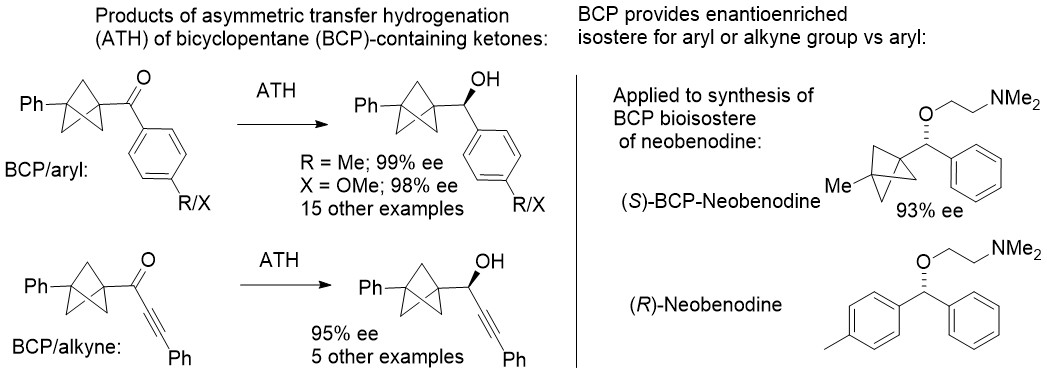
161) Enantioselective Synthesis of Bicyclopentane-Containing Alcohols via Asymmetric Transfer Hydrogenation
Vijyesh K. Vyas, Guy J. Clarkson, Martin Wills, Org. Lett. 2021, 23, 3179–3183. asap published 5th April 2021. ACS articles on demand link: https://pubs.acs.org/articlesonrequest/AOR-PUXJIXHP8IPN9KEZI9ES
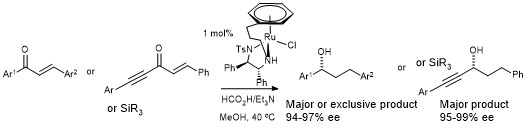
160) Asymmetric Transfer Hydrogenation of Unsaturated Ketones; Factors influencing 1,4- vs 1,2- regio- and enantioselectivity, and alkene vs alkyne directing effects. Thomas H. Hall, Hannah Adams, Vijyesh K. Vyas, K. L. Michael Chu and Martin Wills, Tetrahedron, 2021, article number 131771.
2016-2020
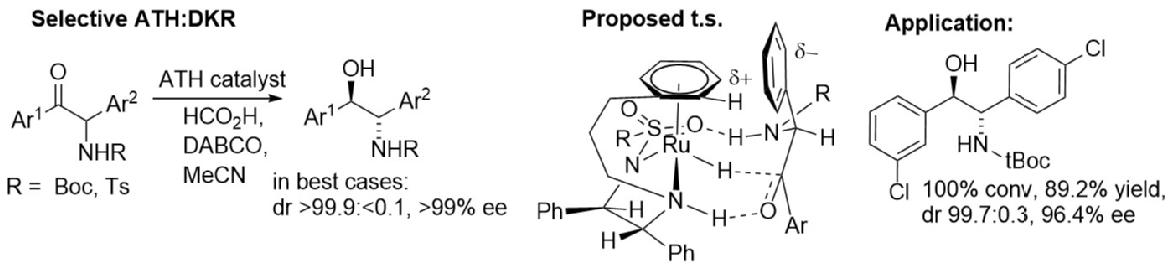
159) Asymmetric Transfer Hydrogenation: Dynamic Kinetic Resolution of alpha;-Amino Ketones, Shweta K. Gediya, Guy J. Clarkson, and Martin Wills, J.Org. Chem. 2020, 85, 11309-11330. ASAP 19th August 2020. ACS articles on demand link. https://pubs.acs.org/doi/10.1021/acs.joc.0c01438.
Review 35) 'A diversity of recently reported methodology for asymmetric imine reduction', Jonathan Barrios-Rivera, Yingjian Xu, Martin Wills and Vijyesh Vyas, Organic Chemistry Frontiers 2020, 7, 3312-3342. accepted 2nd September 2020, first published 3rd September 2020.
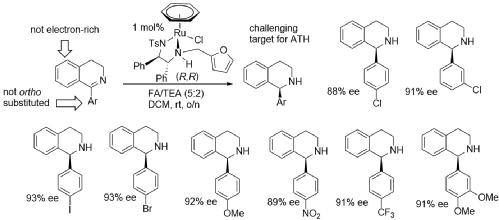
158) Asymmetric Transfer Hydrogenation of Unhindered and Non-Electron-Rich 1Aryl Dihydroisoquinolines with High Enantioselectivity, Jonathan Barrios-Rivera, Yingjian Xu, Martin Wills, Org. Lett. 2020, 22, 6283-6287. DOI 10.1021/acs.orglett.0c02034. ASAP 4th August 2020. This ACS articles on demand link gives up to
50 reprints in the first 12 months
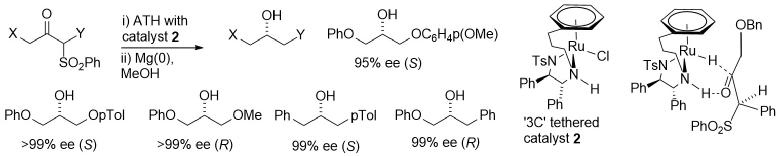
157) The sulfone group as a versatile and removable directing group for the asymmetric transfer hydrogenation of ketones, Vijyesh K. Vyas, Guy J. Clarkson and Martin Wills, Angew Chem. Int. Ed. 2020, 59, 14265-14269. Accepted article online 28th May 2020.
156) Asymmetric Transfer Hydrogenation of alpha-Hydroxyphenyl Ketones: Utilizing Directing Effects That Optimize the Asymmetric Synthesis of Challenging Alcohols, Ye Zheng, Guy J. Clarkson and Martin Wills, Org. Lett. 2020, 22, 3717-3721. ASAP from 16/4/20. Featured in Org. Chem. Highlights: Enantioselective Synthesis of Alcohols and Amines.
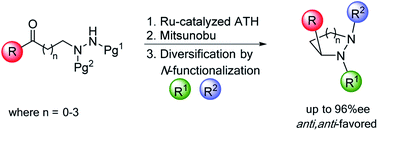
155) Readily Accessible sp3-Rich Cyclic Hydrazine Frameworks Exploiting Nitrogen Fluxionality, Conor Dean, Sundaram Rajkumar, Stefan Roesner, Nessa Carson, Guy James Clarkson, Martin Wills, Matthew L Jones and Mike Shipman* Chemical Science, 2020, 11, 1636 - 1642. Accepted on 23 Dec 2019 and first published on 02 Jan 2020. The lead author is Prof Mike Shipman and the paper describes his group's work on the development of routes to enantiomerically-enriched cyclic hydrazine.
Review 34) 'Applications of N′-monofunctionalised TsDPEN derivatives in asymmetric catalysis' Jonathan Barrios-Rivera, Yingjian Xu and Martin Wills, Org. Biomol. Chem. 2019, 17, 1301-1321, first published on 04 Jan 2019.
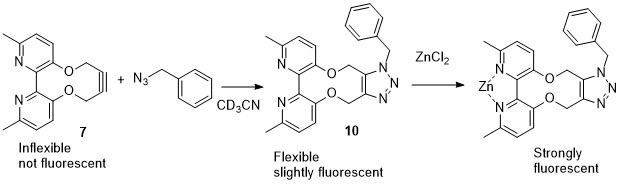
154) A strained alkyne-containing bipyridine reagent; synthesis, reactivity and fluorescence properties, Sam Forshaw, Richard C. Knighton, Jami Reber, Jeremy S. Parker, Nikola P. Chmel and Martin Wills, RSC Advances, 2019, 9, 36154-36161. Open Access from the RSC website. Selected for the themed collection on 'Contributions from the Chemical Industry' September 2021.
153) (S)-(-)-Fluorenylethylchloroformate (FLEC); preparation using asymmetric transfer hydrogenation and application to the analysis and resolution of amines, Mohammad A. Amin, Michelle A. Camerino, Simon J. Mountford, Xiao Ma, David T. Manallack, David K. Chalmers, Martin Wills,* and Philip E. Thompson*, Tetrahedron 2019, 75, Article Number 130591, accepted 2nd September 2019. https://www.sciencedirect.com/science/article/pii/S0040402019309305?via%3Dihub.
45 mg of catalyst generates, after recrystallisation, 3.80g of product in >97% ee (72% yield):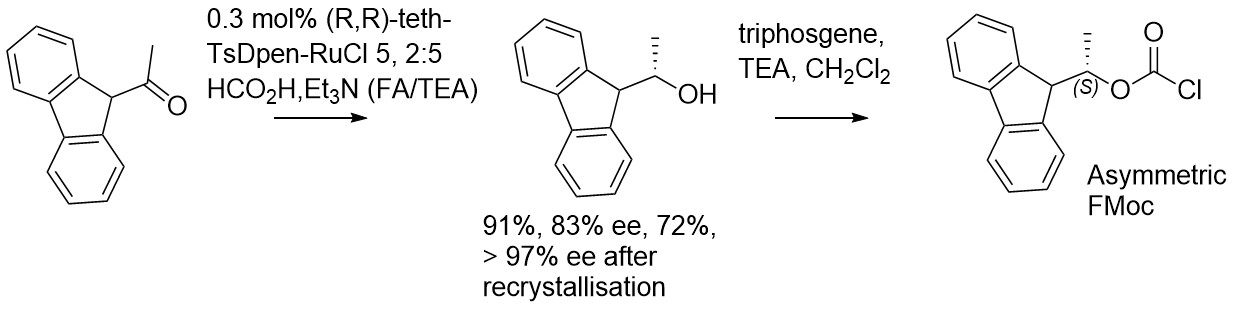
152) Asymmetric Ruthenium Tricarbonyl Cyclopentadienone Complexes; Synthesis and Application to Asymmetric Hydrogenation of Ketones Alessandro Del Grosso, Guy J. Clarkson and Martin Wills Inorganica Chimica Acta, 2019, 496, article 119043, published online 31/7/2019. Open access from the Elsevier website.
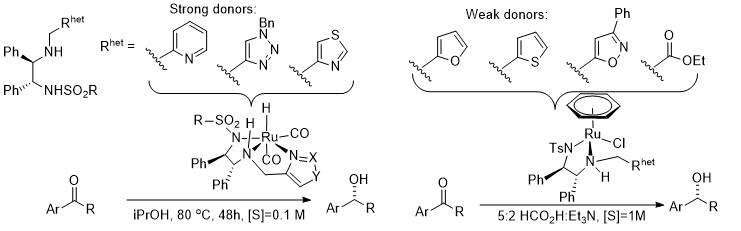
151) Probing the effects of heterocyclic functionality in [(benzene)Ru(TsDPENR)Cl] catalysts for Asymmetric Transfer Hydrogenation. Jonathan Barrios-Rivera,† Yingjian Xu*‡ and Martin Wills * Org. Lett. 2019, 21, 7223-7227. ASAP accepted 26/7/2019. Results from a joint project with GoldenKeys High-tech Materials Co., Ltd., China.
Featured on front cover.
150) Synthesis and Reactivity of a Bis-Strained Alkyne Derived from 1,1′-Biphenyl-2,2′,6,6′-tetrol
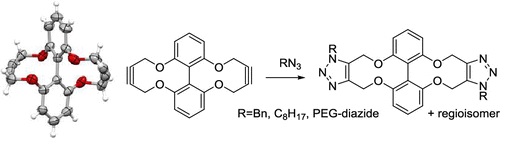
Richard C. Knighton, Krishna Sharma, Naomi S. Robertson, David R. Spring and Martin Wills, ACS Omega, 2019, 4, 2160–2167. A “double strained alkyne” was been prepared and evaluated in strain-promoted azide-alkyne cycloaddition reactions with azides. Accepted January 10th 2019. Open access from the ACS website. PMCID# PMC6648819 through ACS' Certified Deposit program.
149) 'Synthesis and cycloaddition reactions of strained alkynes derived from 2,2′-dihydroxy-1,1′-biaryls',
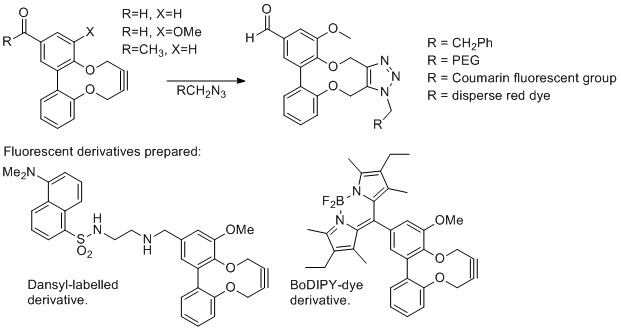
Anish Mistry, Richard C. Knighton, Sam Forshaw, Zakaria Dualeh, Jeremy S. Parker and Martin Wills, Org. Biomol. Chem. 2018, 16, 8965 - 8975. DOI: 10.1039/C8OB01768A. Published online 12/11/2018. Open access from the RSC website.
A series of strained alkynes, based on the 2,2′-dihydroxy-1,1′-biaryl structure, were prepared in a short sequence from readily-available starting materials.
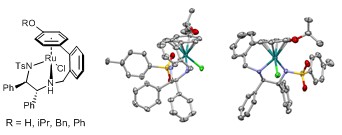
148) Asymmetric transfer hydrogenation of acetophenone derivatives using 2-benzyl-tethered ruthenium(II)/TsDPEN complexes bearing h6-(p-OR)(R=H,iPr,Bn,Ph) ligands. Richard C. Knighton, Vijyesh K. Vyas, Luke H. Mailey, Bhalchandra M. Bhanage, Martin Wills, J. Organomet. Chem. 2018, 875, 72-79. Click here for access to the article before 6th November 2018! Several new tethered catalysts are formed via arene-exchange and demonstrate excellent activity in ketone reduction.
147) Unravelling the Photoprotection Properties of Mycosporine Amino Acid Motifs
Jack M. Woolley, Michael Staniforth, Michael D. Horbury, Gareth W. Richings, Martin Wills , and Vasilios G. Stavros*, J. Phys. Chem. Lett., 2018, 9, 3043–3048. Open access via the ACS website. DOI: 10.1021/acs.jpclett.8b00921. Publication Date (Web): May 11, 2018.
146) 'Transfer Hydrogenation and Antiproliferative Activity of Tethered Half-Sandwich Organoruthenium Catalysts' Feng Chen, Isolda Romero-Canelón, Joan J. Soldevila-Barreda, Ji-Inn Song, James P. C. Coverdale, Guy J. Clarkson, Jana Kasparkova , Abraha Habtemariam, Martin Wills, Viktor Brabec and Peter J. Sadler, Organometallics 2018, 37,1555–1566. Open access via the ACS website. Publication Date (Web): April 23, 2018 (Article) DOI: 10.1021/acs.organomet.8b00132.
145) 'Exploitation of differential electronic densities for the stereoselective reduction of ketones bearing a masked amino surrogate' Renta Jonathan Chew* and Martin Wills* J. Catal. 2018, 361, 40-44.
144) 'Ruthenium-Catalyzed Asymmetric Reduction of Isoxazolium Salts: Access to Optically Active Δ4-Isoxazolines' Renta Jonathan Chew* and Martin Wills* J. Org. Chem. 2018, 83, 2980–2985. Article ASAPDOI: 10.1021/acs.joc.7b03229. Publication Date (Web): February 6, 2018.
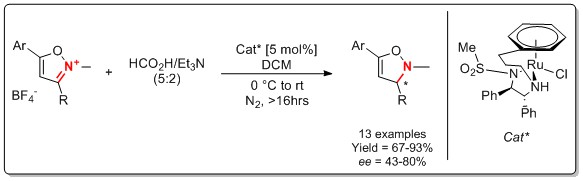
143) An alternative route to tethered Ru(II) transfer hydrogenation catalysts, Roy Hodgkinson, Václav Jurčík, Hans Nedden, Andrew Blackaby, Martin Wills, Tetrahedron Lett. 2018, 59, 930-933. Accepted 23 January 2018, Available online 1 February 2018. Link to shared article valid until 30/3/18. https://doi.org/10.1016/j.tetlet.2018.01.071.
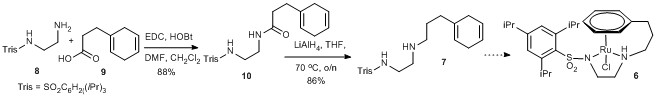
142) ‘Combining Electronic and Steric Effects To Generate Hindered Propargylic Alcohols in High Enantiomeric Excess’, Vijyesh K. Vyas, Richard C. Knighton, Bhalchandra M. Bhanage, and Martin Wills, Org. Lett. 2018, 20, 975–978. Publication Date (Web): January 31, 2018. DOI: 10.1021/acs.orglett.7b03884. A remarkable selectivity pattern for reduction of challenging substrates. Open access from the ACS website:
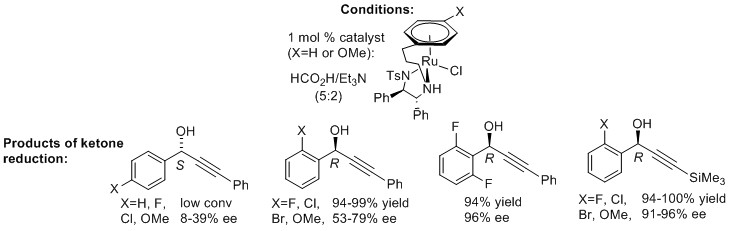
141) Asymmetric Transfer Hydrogenation by Synthetic Catalysts in Cancer Cells, James P. C. Coverdale, Isolda Romero-Canelón, Carlos Sanchez-Cano, Guy J. Clarkson, Abraha Habtemariam, Martin Wills & Peter J. Sadler, Nature Chemistry 2018, 10, 347–354, published online 8th January 2018. https://www.nature.com/articles/nchem.2918
abstract : '...Here we use highly stable chiral half-sandwich organometallic Os(II) arene sulfonyl diamine complexes, [Os(arene)(TsDPEN)] (TsDPEN, N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine), to achieve a highly enantioselective reduction of pyruvate, a key intermediate in metabolic pathways. Reduction is shown both in aqueous model systems and in human cancer cells, with non-toxic concentrations of sodium formate used as a hydride source...'
140) Synthesis and Applications to Catalysis of Novel Cyclopentadienone Iron Tricarbonyl Complexes,
Alessandro Del Grosso, Alexander E. Chamberlain, Guy J. Clarkson and Martin Wills*, Dalton Trans. 2018, 47, 1451-1470. Published online (advance article) 5th January 2018, DOI 10.1039/C7DT03250A. Open access from the RSC website.
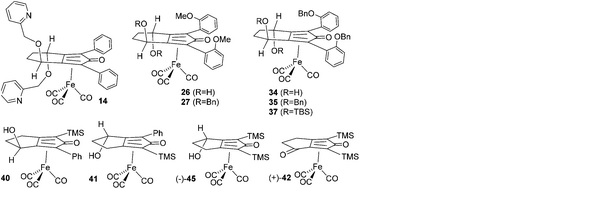
Rina Soni, Katherine E. Jolley, Silvia Gosiewska, Guy J. Clarkson, Zhijia Fang, Thomas H. Hall, Ben N. Treloar, Richard C. Knighton, and Martin Wills, Organometallics 2018, 37, 48–64. Publication Date (Web): December 20, 2017 (Article)
DOI: 10.1021/acs.organomet.7b00731
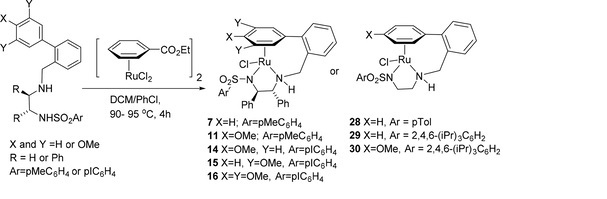
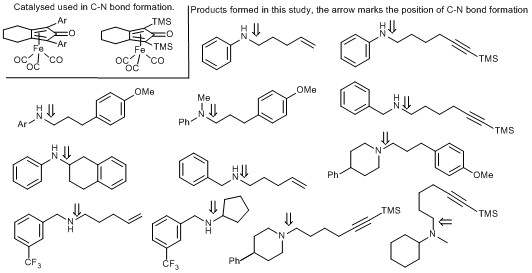
138) Use of (Cyclopentadienone)iron Tricarbonyl Complexes for C–N Bond Formation Reactions between Amines and Alcohols, Thomas J. Brown, Madeleine Cumbes, Louis J. Diorazio, Guy J. Clarkson and Martin Wills, J. Org. Chem. 2017, 82, 10489–10503. Publication Date (Web): September 18, 2017, 10.1021/acs.joc.7b01990. Open access from ACS website.
Alessandro Del Grosso, Lavrentis-Dimitrios Galanopoulos, Cookson K. C. Chiu, Guy J. Clarkson, Peter B. O′ Connor and Martin Wills* Org. Biomol.Chem. 2017, 15, 4517 - 4521: Open access from RSC website: http://pubs.rsc.org/en/content/articlelanding/2017/ob/c7ob00991g#!divAbstract
136) Asymmetric Transfer Hydrogenation of 1,3-Alkoxy/Aryloxy Propanones Using Tethered Arene/Ru(II)/TsDPEN Complexes, Sam Forshaw, Alexander J. Matthews, Thomas J. Brown, Louis J. Diorazio, Luke Williams and Martin Wills*. Org. Lett, 2017, 19, 2789-2792. (collaboration with AstraZeneca). Open access from ACS website.
DOI: 10.1021/acs.orglett.7b00756, Publication Date (Web): May 16, 2017 pp 2789–2792.
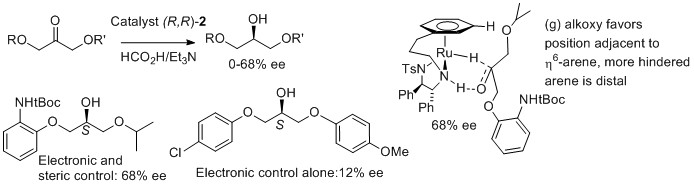
Review 33) 'The Development of Phosphine-Free "Tethered" Ruthenium(II) Catalysts for the Asymmetric Reduction of Ketones and Imines', Hans G. Nedden, Antonio Zanotti-Gerosa and Martin Wills. Jointly authored with Johnson Matthey, Cambridge. First published: 15 August 2016. The Chemical Record, 2016, 16, 2619–2639. Open-access.
135) 'The contrasting catalytic efficiency and cancer cell antiproliferative activity of stereoselective organoruthenium transfer hydrogenation catalysts' Ying Fu, Carlos Sanchez-Cano, Rina Soni, Isolda Romero-Canelon, Jessica M. Hearn, Zhe Liu, Martin Wills and Peter J. Sadler, Dalton Trans., 2016, 45, 8367-8378. Advance Article DOI: 10.1039/C6DT01242F, Paper First published online : 25 Apr 2016.
The following complexes were evaluate for anticancer activity and the N-Me derivatives showed excellent activity:
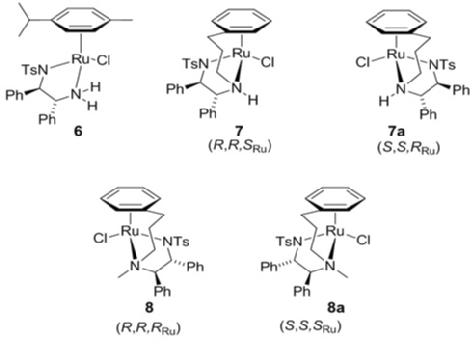
134) Iron cyclopentadienone complexes derived from C2-symmetric bis-propargylic alcohols; preparation and applications to catalysis, Roy C Hodgkinson, Alessandro Del Grosso, Guy J Clarkson and M Wills, Dalton Trans., 2016, 45, 3992 - 4005. DOI: 10.1039/C5DT04610F
Complexes below were applied to asymmetric transfer hydrogenation and pressure hydrogenation of ketones:
Review 32) 'Imino Transfer Hydrogenation Reactions' ReviewTopics in Current Chemistry, April 2016, 374:14
First online: 14 March 2016. http://link.springer.com/article/10.1007/s41061-016-0013-7?wt_mc=alerts.TOCjournals
A review of methods for imine reduction, including asymmetric reductions.
2011-2015
133) Practical Access to Planar Chiral 1,2-(α-Ketotetramethylene)- ferrocene by Non-Enzymatic Kinetic Resolution and Conclusive Confirmation of its Absolute Configuration, Ruixia Liu, Gang Zhou, Thomas H. Hall, Guy J Clarkson, Martin Wills,* and Weiping Chen, Adv. Synth. Catal. 2015, 357, 3453-3457.
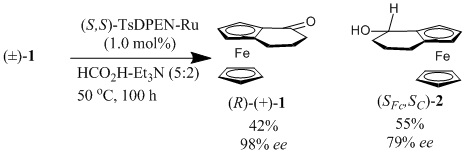
132) 'N-Functionalised TsDPEN catalysts for asymmetric transfer hydrogenation; synthesis and applications', Rina Soni, Thomas H. Hall, David J. Morris, Guy J. Clarkson, Matthew R. Owen, Martin Wills, Tetrahedron Lett. 2015, 56, 6397-6401. Available online 30 September 2015. Click on this link for free access until 16th December 2015.
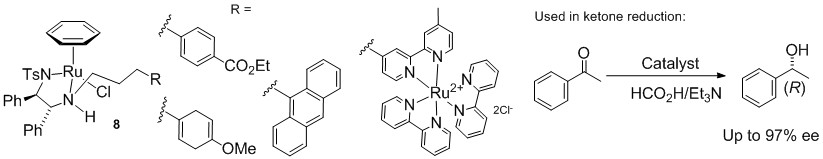
131) 'Asymmetric reduction of electron-rich ketones with tethered Ru(II)/TsDPEN catalysts using formic acid/triethylamine or aqueous sodium formate', Rina Soni, Thomas H Hall, Benjamin P Mitchell, Matthew R Owen, and Martin Wills J. Org. Chem. 2015, 80,
6784−6793. ASAP. June 12, 2015 (Article)DOI: 10.1021/acs.joc.5b00990.
Open access from ACS website.
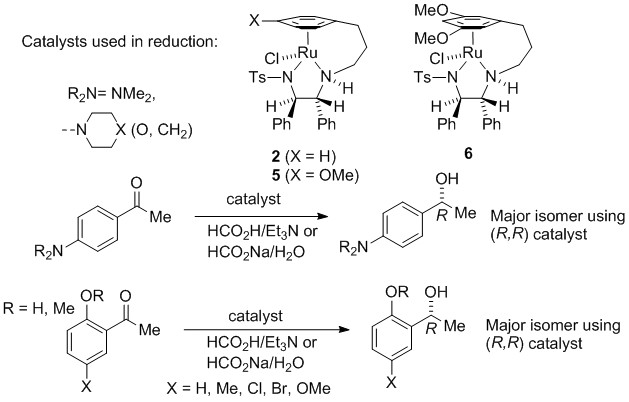
130) Easy To Synthesize, Robust, Organo-Osmium Asymmetric Transfer Hydrogenation Catalysts, James P. C. Coverdale,Dr. Carlos Sanchez-Cano,Dr. Guy J. Clarkson,Dr. Rina Soni,Prof. Dr. Martin Wills* and Prof. Dr. Peter J. Sadler*, Chemistry; A European Journal, 2015, 21, 8043–8046. Open access from the website. Article first published online: 8 APR 2015 DOI: 10.1002/chem.201500534.
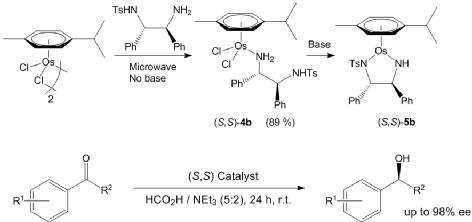
129) 'CN Bond Formation between Alcohols and Amines Using an Iron Cyclopentadienone Catalyst' Andrew J. Rawlings, Louis J. Diorazio, and Martin Wills, Org.Lett. 2015, 17, 1086-1089. ASAP DOI: 10.1021/ol503587n published online 17th February 2015.
Open access from ACS website.
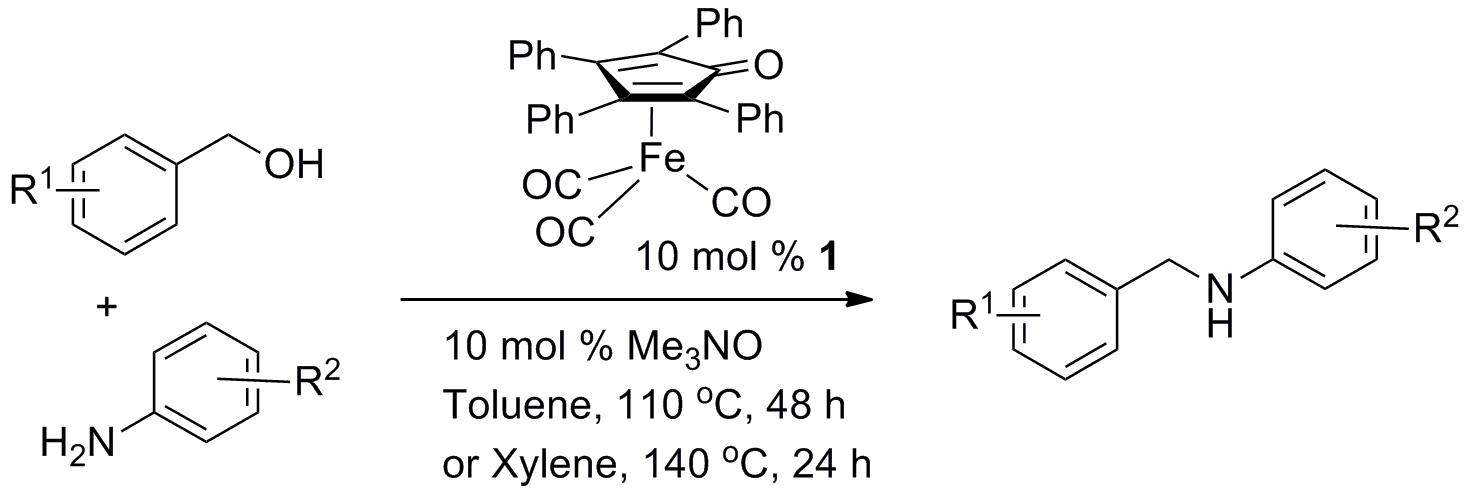
128) 'Tethered Ru(II) catalysts containing a Ru-I bond', Katherine E Jolley, Guy J. Clarkson and Martin Wills, J. Organomet. Chem. 2015, 776, 157-162.
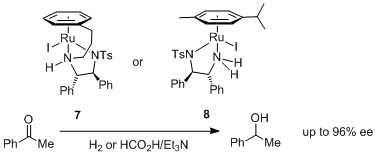
127) 'Synthesis and Catalytic Applications of an Extended Range of Tethered Ruthenium(II)/η6-Arene/Diamine Complexes', Roy Hodgkinson, Václav Jurčík, Antonio Zanotti-Gerosa, Hans Günter Nedden, Andrew Blackaby, Guy J. Clarkson, and Martin Wills, Organometallics 2014, 33, 5517-5524.
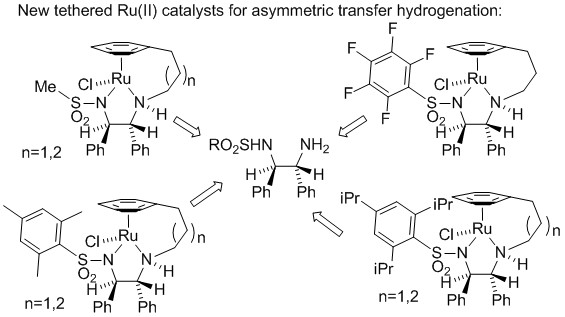
126) 'Synthesis and reduction reactions of pyridones and 5-acyl-2-methoxypyridines'
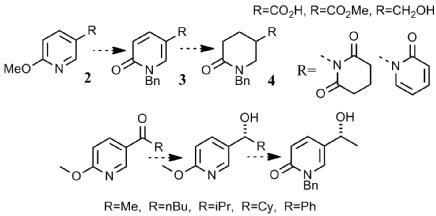
Alexander A. Bisset, Allan Dishington, Teyrnon Jones, Guy J. Clarkson, Martin Wills, Tetrahedron 2014, 70, 7207-7220.
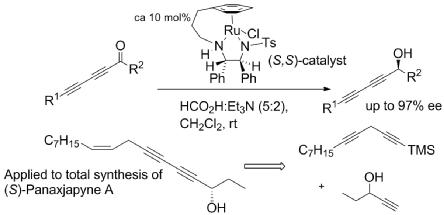
125) 'Asymmetric Reduction of Diynones and the Total Synthesis of (S)-Panaxjapyne A', Zhijia Fang and Martin Wills, Organic Letters, 2014, 16, 374-377. Publication Date (Web): December 30, 2013. DOI: 10.1021/ol4032123.
124) 'Asymmetric reduction of 2,2-dimethyl-6-(2-oxoalkyl/oxoaryl)-1,3-dioxin-4-ones and application to the synthesis of (+)-yashabushitriol' Zhijia (Amphi) Fang, Guy J. Clarkson, Martin Wills, Tetrahedron Lett. 2013, 54, 6834-6837 DOI: 10.1016/j.tetlet.2013.10.010, online from Oct 10th 2013. Highlighted in SYNFACTS (http://www.thieme-connect.com/ejournals/toc/synfacts ).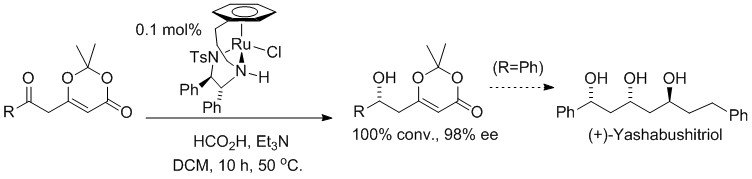
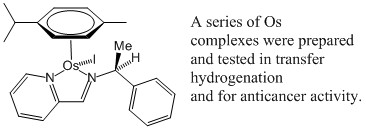
123) Mirror-Image Organometallic Osmium Arene Iminopyridine Halido Complexes Exhibit Similar Potent Anticancer Activity, Ying Fu, Rina Soni, María J. Romero, Ana M. Pizarro, Luca Salassa, Guy J. Clarkson, Jessica M. Hearn, Abraha Habtemariam, Martin Wills, Peter J. Sadler, Chem. Eur. J. 2013, 19, 15199-15209. published online: 23 SEP 2013. DOI: 10.1002/chem.201302183. Highlighted on the ChemistryViews website. The short news article is available at http://www.chemistryviews.org/details/ezine/5163551/Mirror_Images_Chiral_Affects_in_Osmium_Complexes.html. On WRAP.
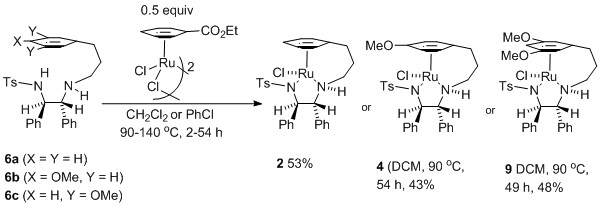
122) 'Direct Formation of Tethered Ru(II) Catalysts Using Arene Exchange', Rina Soni, Katherine E Jolley, Guy J. Clarkson and Martin Wills, Org. Lett. 2013, 15, 5110–5113. DOI: 10.1021/ol4024979. Publication Date (Web): September 26, 2013. You can download this via the ACS articles on demand system using this link:
http://pubs.acs.org/articlesonrequest/AOR-se2t8w5DJp8U39UNM8y9.
Open access from ACS website.
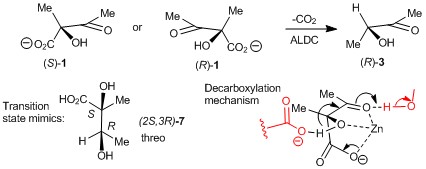
121) 'Structure and Mechanism of Acetolactate Decarboxylase', Victoria A. Marlow, Dean Rea, Shabir Najmudin, Martin Wills, and Vilmos Fülöp, ACS Chemical Biology, 2013, 8, 2339-2344. DOI: 10.1021/cb400429h. Publication Date (Web): August 14, 2013 (Articles). The mechanism of acation of acetolactate decarboxylase was investigated using X-ray crystallography with co-ordinated mimics and kinetic studies of transition state inhibitors. WRAP.
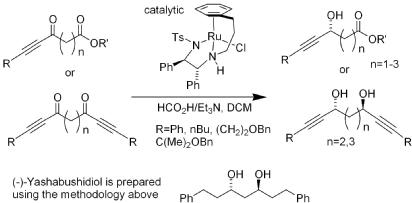
120) 'Asymmetric Transfer Hydrogenation of Functionalized Acetylenic Ketones' Zhijia Fang and Martin Wills, J. Org. Chem. 2013, 78, 8594–8605. published online 15/8/13.
119) ‘Use of triazole-ring formation to attach a Ru/TsDPEN complex for asymmetric transfer hydrogenation to a soluble polymer.’ Charlotte M. Zammit and Martin Wills, Tetrahedron: Asymmetry 2013, 24, 844-852. DOI: 10.1016/j.tetasy.2013.05.022.

118) ‘Use of tridentate TsDPEN/pyridine ligands in ruthenium-catalysed asymmetric reduction of ketones’. Moftah O. Darwish, Alistair Wallace, Guy J. Clarkson and Martin Wills, Tetrahedron Lett. 2013, 54, 4250-4253. http://dx.doi.org/10.1016/j.tetlet.2013.05.141.
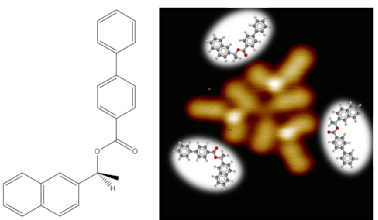
117) ’Dissociation and hierarchical assembly of chiral esters on metallic surfaces.’ Ben Moreton, Zhijia Fang, Martin Wills and Giovanni Costantini, Chem. Commun. 2013, 49, 6477-6479. A paper from our collaboration with the Costantini group, which featured on the cover of the journal. The Scanning Tunneling Microscope image below shows the enantiomerically-pure ester on a gold surface. The absolute configuration of the molecule is directly observed.
Review 31) ‘Asymmetric catalysis using iron complexes – ‘Ruthenium Lite’?’ M. Darwish and M. Wills, Catal. Sci. Technol. 2012, 2 (2), 243 - 255; http://xlink.rsc.org/?doi=C1CY00390A.
116) 'Synthesis and asymmetric hydrogenation of (3E)-1-benzyl-3-[(2-oxopyridin-1(2H)-yl)methylidene]piperidine-2,6-dione', Alexander A. Bisset, Akira Shiibashi, Jasmine L. Desmond, Allan Dishington, Teyrnon Jones, Guy J. Clarkson, Takao Ikariya and Martin Wills' Chem. Commun. 2012, 48, 11978 - 11980, http://xlink.rsc.org/?doi=C2CC36807B. 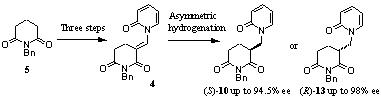
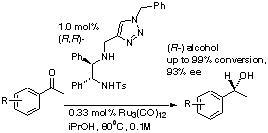
115) 'Application of Ruthenium Complexes of Triazole-Containing Tridentate Ligands to Asymmetric Transfer Hydrogenation of Ketones' Tarn C. Johnson, William G. Totty and Martin Wills, Organic Letters, 2012, 14, 5230–5233. (Highlighted in Synfacts 2013 9(1), 0062).
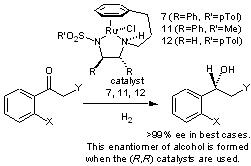
114) 'Application of Tethered Ruthenium Catalysts to Asymmetric Hydrogenation of ketones, and the Selective Hydrogenation of Aldehydes', Katherine E. Jolley, Antonio Zanotti-Gerosa Fred Hancock, Alan Dyke, Damian M. Grainger, Jonathan A. Medlock, Hans G. Nedden, Jacques J. M. Le Paih, Stephen J. Roseblade, A. Seger, V. Sivakumar, David J Morris and Martin Wills, Adv. Synth. Catal. 2012, 354, 2545-2555.
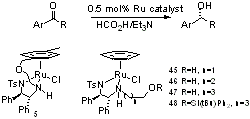
113) ''Ether- Tethered Ru(II)/TsDPEN Complexes; Synthesis and Applications to Asymmetric Transfer Hydrogenation' Vimal Parekh, James A Ramsden and Martin Wills, Catal. Sci. Technol., 2012, 2 (2), 406-414. DOI:10.1039/C1CY00364J. Published online: http://xlink.rsc.org/?doi=C1CY00364J. WRAP.
A Ru(II) catalyst containing an ether-tether exhibits excellent activity and versatility in asymmetric transfer hydrogenation of ketones and an imine.
112) '‘Developing asymmetric iron and ruthenium-based cyclone complexes; complex factors influence the asymmetric induction in the transfer hydrogenation of ketones’ Jonathan P. Hopewell, José E. D. Martins, Tarn C. Johnson, Jamie Godfrey and Martin Wills, Org. Biomol. Chem. 2012, 10, 134-145, DOI:10.1039/C1OB06010D. WRAP.
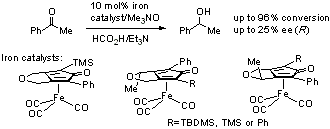
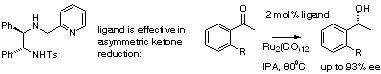
111) 'Application of Proline-Functionalised 1,2-Diphenylethane-1,2-Diamine (DPEN) in Asymmetric Transfer Hydrogenation of Ketones.' Charles V. Manville, Gordon Docherty, Ranbir Padda and Martin Wills, Eur. J. Org. Chem. 2011, 6893–6901.
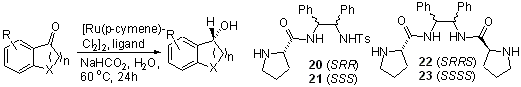
110) 'An Unexpected Directing Effect in the Asymmetric Transfer Hydrogenation of a,a-Disubstituted Ketones', Rina Soni, John-Michael Collinson, Guy C. Clarkson, and Martin Wills, Organic Letters 2011, 13, 4304–4307. Highlighted in Synfacts. available here:
109) ‘(Cyclopentadienone)iron-Shvo complexes; synthesis and applications to hydrogen transfer reactions’ Tarn C. Johnson, Guy J. Clarkson and Martin Wills, Organometallics 2011, 30, 1859-1868. (om-2010-01101r). http://pubs.acs.org/articlesonrequest/
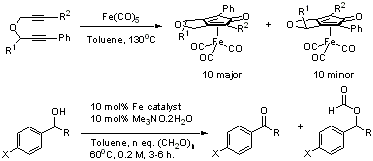
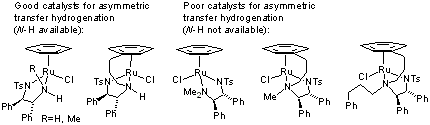
108) 'The Importance of the N-H Bond in Ru/TsDPEN Complexes for Asymmetric Transfer Hydrogenation of Ketones and Imines', Rina Soni, Fung Kei Cheung,Guy C. Clarkson, Jose E. D. Martins, Mark A. Grahamand Martin Wills, Org. Biomol. Chem. 2011, 9, 3290-3294, DOI: 10.1039/c1ob05208j.
2010-2006
107) 'Gold-Catalysed Cyclic Ether Formation from Diols', Xiaolu Jiang, Emma K. London, David J. Morris, Guy J. Clarkson and Martin Wills, Tetrahedron, 2010, 66, 9828-9834.
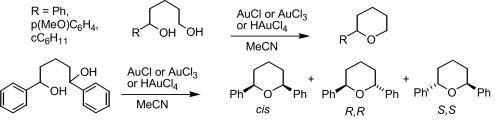
106) 'Applications of N′-alkylated derivatives of TsDPEN in the asymmetric transfer hydrogenation of C=O and C=N bonds', José E.D. Martins, Miguel A. Contreras Redondo and Martin Wills, Tetrahedron: Asymmetry, 2010, 21, 2258-2264.

105) 'Synthesis and use of a Stable Aminal Derived from TsDPEN in Asymmetric Organocatalysis', Silvia Gosiewska, Rina Soni, Guy J. Clarkson and Martin Wills, Tetrahedron Lett. 2010, 51, 4214-4217. WRAP.
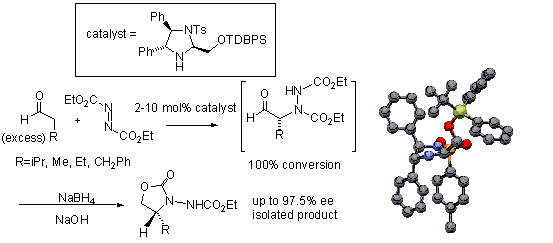
104) 'Inhibition of Prolyl Oligopeptidase using a Synthetic Unnatural Dipeptide', Daurgirdas T. Racys, Dean Rea, Vilmos Fulop and Martin Wills, Bioorg Med. Chem. 2010, 18, 4775-4782. WRAP.
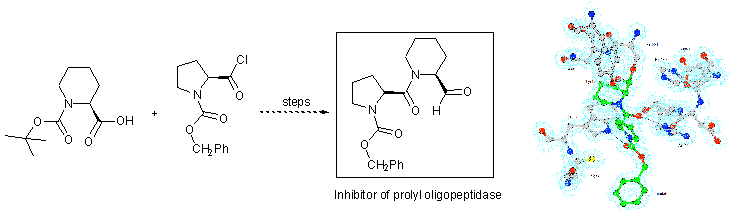
103) 'Asymmetric transfer hydrogenation of quinolines using tethered Ru(II) catalysts', Vimal Parekh, James A. Ramsden and Martin Wills, Tetrahedron: Asymmetry, 2010, 21, 1549-1556.
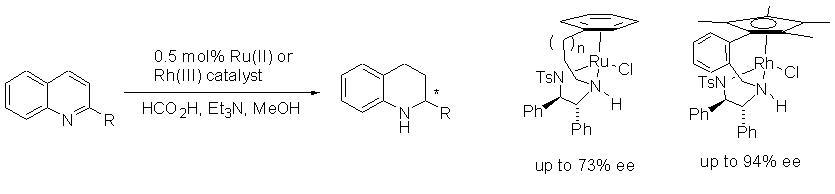
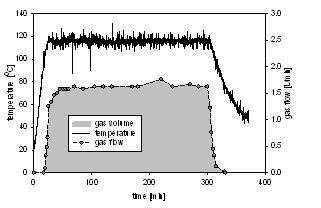
102) 'A Continuous-Flow Method for the Generation of Hydrogen from Formic Acid', Artur Majewski, David J. Morris, Kevin Kendall and Martin Wills, ChemSusChem, 2010, 3, 431-434.
101) 'Kinetic and structural studies on 'tethered'Ru(II) arene ketone reduction catalysts' Fung Kei (Kathy) Cheung, Adam J. Clarke, Guy J. Clarkson, David J. Fox, Mark A. Graham Changxue Lin, Andriana Lorente Crivillé and Martin Wills, Dalton Trans. 2010, 39, 1395 - 1402. WRAP.
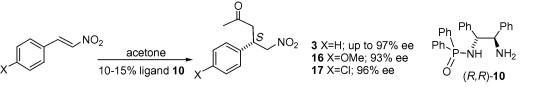
100) 'Asymmetric organocatalysis of the addition of acetone to 2-nitrostyrene using N-diphenylphosphine-1,2-diphenylethane-1,2-diamine (PODPEN)', David J. Morris, A. Simon Partridge, Charles V. Manville, Daurgirdas T. Racys, Gary Woodward, Gordon Docherty and Martin Wills, Tetrahedron Lett. 2010, 51, 209-212. WRAP.
(note added 27/11/12; PODPEN was the best ligand in a Warfarin synthesis; J. Dong and D.-M. Du, Org. Biomol. Chem. 2012, 10, 8125-8131).
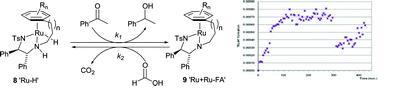
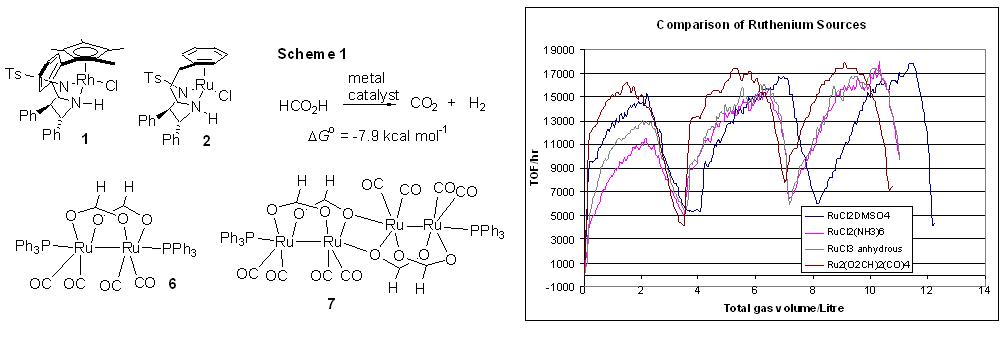
99) 'Insights into hydrogen generation from formic acid using Ru complexes', David J. Morris, Guy J. Clarkson and Martin Wills, Organometallics, 2009, 28, 4133-4140. (om900099u).
98) 'Ir(III) complexes of diamine ligands for asymmetric ketone hydrogenation', José E. D. Martins and Martin Wills, Tetrahedron 2009, 65, 5782-5786. WRAP.
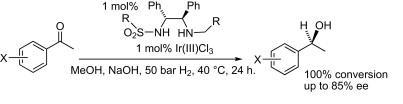
97) ‘Ru(II) Complexes of N-Alkylated TsDPEN Ligands in Asymmetric Transfer Hydrogenation of Ketones and Imines’ José E. D. Martins, Guy J. Clarkson and Martin Wills, Org. Lett. 2009, 11 847-850. (ol802801p).
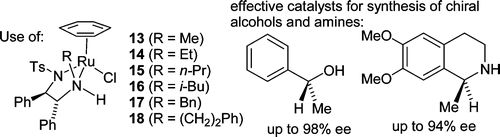
96) ‘Asymmetric hydrogenation of ketones using Ir(III) complexes of N-alkyl-N’-tosyl-1,2-ethanediamine ligands.', José E. D. Martins, David J. Morris and Martin Wills, Tetrahedron Lett. 2009, 50, 688-692.
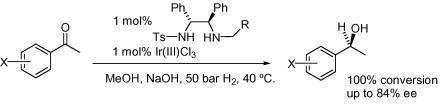
95) ‘Further ‘tethered’ Ru(II) catalysts for asymmetric transfer hydrogenation (ATH) of ketones; the use of a benzylic linker and a cyclohexyldiamine ligand’, José E. D. Martins, David J. Morris, Bhavana Tripathi and Martin Wills, J. Organomet. Chem., 2008, 693, 3527-3532.
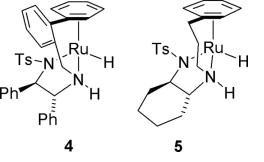
94) ‘Asymmetric Transfer Hydrogenation of C=O and C=N bonds by Tethered Rh(III) Catalysts’ Martin Wills, Daljit S. Matharu and José E. D. Martins, Chemistry; An Asian Journal, 2008, 3, 1374-1383.
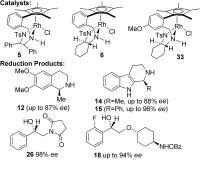
93) ‘Synthesis of a series of novel N,N-dialkyl-TsDPEN ligands and their application to enantioselective addition of dialkylzinc to benzaldehyde’, José E. D. Martins and Martin Wills, Tetrahedron: Asymmetry, 2008, 19, 1250-1255.
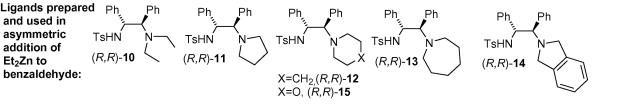
92) ‘An investigation into the tether length and substitution pattern of arene-substituted complexes for asymmetric transfer hydrogenation of ketones’, Fung K. Cheung, Changxue Lin, Franco Minissi, Adriana Lorente Crivillé, Mark A. Graham, David J. Fox and Martin Wills, Org. Lett. 2007, 9, 4659-4662.
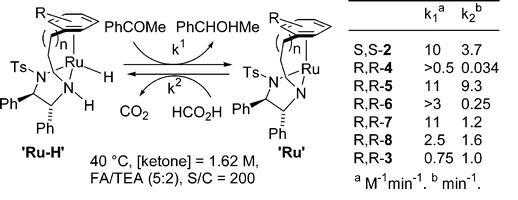
91) ‘Ether-linked’ Organometallic catalysts for ketone reduction reactions’, Fung Kei Cheung, Mark A. Graham,Franco Minissi and Martin Wills, Organometallics, 2007, 26, 5346-51.
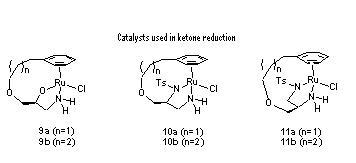
90) ‘An optimised synthetic approach to a chiral derivatising agent and the utilization of a dimerisation reaction in the synthesis of a novel C2-symmetric diphosphine ligand.’ Glynn D. Williams, Charles E. Wade, Guy Clarkson and Martin Wills, Tetrahedron: Asymmetry, 2007, 18, 664-670.
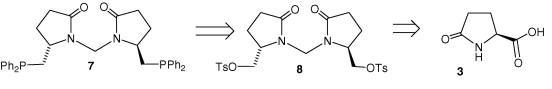
89) ‘The use of a [4+2] cycloaddition reaction for the preparation of a series of ‘tethered’ Ru(II)/diamine and aminoalcohol complexes’, Fung Kei Cheung, Aidan M. Hayes, David J. Morris and Martin Wills, Org. Biomol. Chem. 2007, 5, 1093-1103. (b700744b).
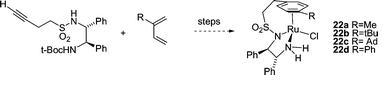
88) ‘Asymmetric opening of the epoxide ring in cyclohexene oxide by thiophenol using homochiral phosphinamide catalysts’, Atsushi Nagasawa, Changxue Lin and Martin Wills, J. Chem. Res. 2007, 1-4.
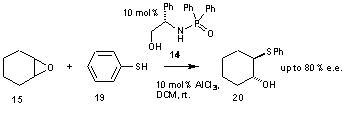
87) ‘Modification of ligand properties of phosphine ligands for C-C and C-N bond-forming reactions’ David J. Morris, Gordon Docherty, Gary Woodward, and Martin Wills, Tetrahedron Lett. 2007, 48, 949-943.
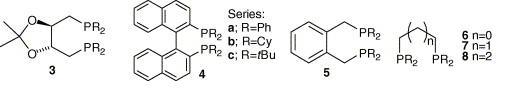
86) ‘Ru(II) complexes of cyclohexane diamine and monodentate phosphorus ligands for asymmetric ketone hydrogenation' Yingjian (Andy) Xu, Gordon F. Docherty,Gary Woodward and Martin Wills, Tetrahedron: Asymmetry, 2006, 17, 2925-2929. Doi:10.1016/j.tetasy.2006.10.036.
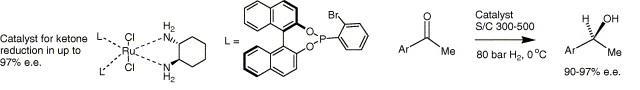
85) ‘The ‘reverse-tethered’ ruthenium (II) catalyst for asymmetric transfer hydrogenation: further applications.’ David J. Morris, Aidan M. Hayes and Martin Wills, J. Org. Chem., 2006, 71, 7035-7044 (jo061154l).
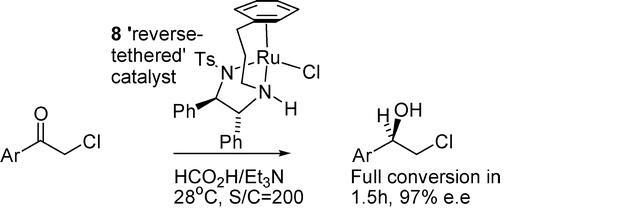
84) “An outstanding catalyst for asymmetric hydrogenation in aqueous solution and formic acid/triethylamine”, Daljit S. Matharu, Guy J. Clarkson, David J. Morris and Martin Wills, Chem. Commun. 2006, 3232-3234.
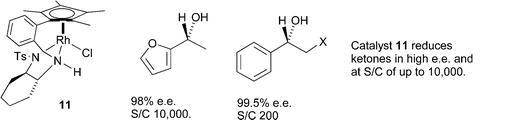
83) “Asymmetric Transfer Hydrogenation of ?? Unsaturated, a-Tosyloxy and a-Substituted Ketones”, Jerome Hannedouche, Philip Peach, David J. Cross,Jennifer A. Kenny, Inderjit Mann, Ian Houson,Lynne Campbell, Tim Walsgrove, and Martin Wills, Tetrahedron, 2006. 62, 1864-76.
2005-2001
82) 'A Stereochemically Well-Defined Rhodium(III) Catalyst for Asymmetric Transfer Hydrogenation of Ketones', Daljit S. Matharu, David J. Morris, Aparecida M. Kawamoto, Guy J. Clarkson, and Martin Wills, Organic Letters, 2005, 7, 5489 – 5491 (ol052559f).
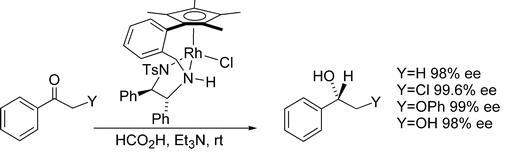
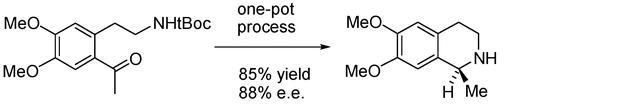
81) “One-pot formation of nitrogen-containing heterocyclic ring systems using a deprotection/cyclisation/asymmetric reduction sequence.”, Glynn D. Williams, Charles E. Wade and Martin Wills, Chem. Commun., 2005, 4735-4737 (B509231).
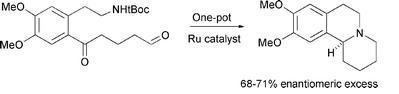
80) "Ruthenium(II) complexes of monodonor ligands; efficient reagents for asymmetric ketone hydrogenation.”, Yingjian (Andy) Xu, Guy Clarkson, Gordon Docherty, Carl North, Gary Woodward and Martin Wills, J. Org. Chem., 2005, 70,8079-8087 (JO051176s).
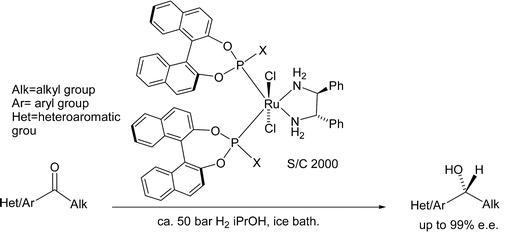
79) “Asymmetric transfer hydrogenation using amino acid derivatives; further studies and a mechanistic proposal”, Aveline S. Y. Yim, and Martin Wills, Tetrahedron, 2005, 61,7994-8004.
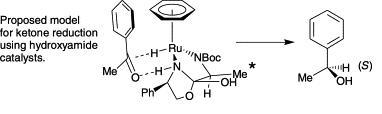
78) "A Class of Ruthenium(II) catalyst for Asymmetric Transfer Hydrogenations of Ketones.", Aidan M. Hayes, David J. Morris, Guy J. Clarkson and Martin Wills, J. Am. Chem. Soc. 2005, 127, 7318-9.
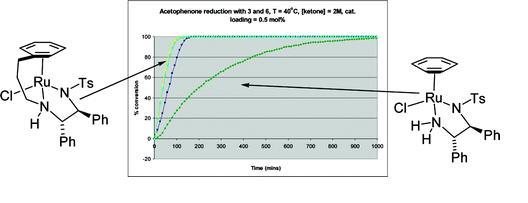
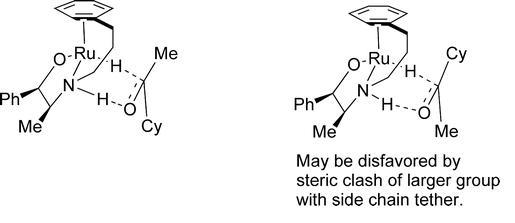
77) ‘"Tethered" Ru(II) Catalysts for Asymmetric Transfer Hydrogenation of Ketones’, Fung Kei (Kathy) Cheung, Aidan M. Hayes, Jerome Hannedouche, Aveline S. Y. Yim, and Martin Wills, J. Org. Chem., 2005, 70, 3188 – 3197.
76) ‘Parameterising matrix-assisted laser desorption/ionisation (MALDI): strategy for matrix-analyte selection and effect of radical co-additives on analyte peak intensities’, Saj Bashir, Roger Mutter, A. E. Giannakopulos, Martin Wills and Peter J Derrick, ANALYTICA CHIMICA ACTA, 2004, 519, 181-187.
75) ‘Synthesis of a poly(amic acid) for application as interphase in high performance thermoplastic composites.’, Liliana B. Nohara, Aparecida M. Kawamoto, Marta F.K. Takahashi, Martin Wills, Evandro L. Nohara and Mirabel C. Rezende, Polimeros: Ciencia e Tecnologia, 2004, 14, 122-128.
74) “Asymmetric Hydrogenation of ketones using a ruthenium(II) catalyst containing BINOL-derived monodonor phosphorus-donor ligands”, Y. Xu, N. W. Alcock, G. J. Clarkson, G. Docherty, G. Woodward and M. Wills, Organic Letters, 2004, 6, 4105-4107. Featured in Anne Thayer article on CHiral Chemistry in Chemical and Engineering news, sept 5th 2005, 40-47.
73) “A Soluble-Polymer System for the Asymmetric Transfer Hydrogenation of Ketones”, Stephanie Bastin, Richard J. Eaves, Christopher W. Edwards, Osamu Ichihara, Mark Whittaker, and Martin Wills, J. Org. Chem., 2004, 69, 5405 – 5412.
72) “ The importance of 1,2-anti-disubstitution in monotosylated diamine ligands for ruthenium(II)-catalysed asymmetric transfer hydrogenation”, Aidan Hayes, Guy Clarkson and Martin Wills, Tetrahedron: Asymmetry, 2004, 15, 2079-84.

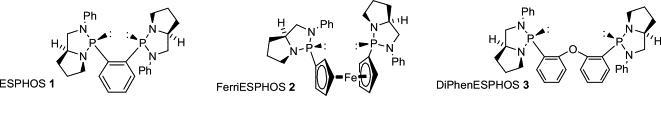
71) “Bis(diazaphospholidine) ligands for asymmetric hydroformylation: use of ESPHOS and derivatives based on ferrocene and diarylether backbones”, Guy J. Clarkson, Jeff R. Ansell, David J. Cole-Hamilton, Peter J. Pogorzelec, John Whittell and Martin Wills, Tetrahedron: Asymmetry, 2004, 15, 1787-92.
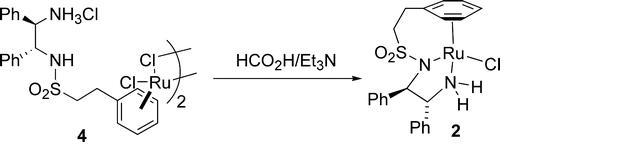
70) “A New Class of ‘Tethered’ Ruthenium(II) Catalysts for Asymmetric Transfer Hydrogenation Reactions”, Jerome Hannedouche, Guy Clarkson and Martin Wills, J. Am. Chem. Soc., 2004, 126, 986-987 (JA0392768).
69) “A New Class of Rh(III) Catalyst Containing an Aminoalcohol Tethered to a Tetramethylcyclopentadienyl Group for Asymmetric Transfer Hydrogenation”, David J. Cross, Ian Houson, Aparecida M. Kawamoto and Martin Wills, Tetrahedron Lett., 2004, 45, 843-846.
68) “Unexpected Formation of a Borated P-Azulene via the Reaction of a Borated Diazaphospholidine with Phenyllithium”, Jeffrey R. Ansell, Nathanial W. Alcock and Martin Wills, J. Chem. Res., 2003, 728-729.
67) “A One-Pot Process for the Enantioselective Synthesis of Amines via Reductive Amination under Transfer Hydrogenation Conditions”, Glynn D. Williams, Richard A. Pike, Charles E. Wade, and Martin Wills, Organic Lett., 2003, 4227-4230.
66) “Synthesis and Application to Asymmetric Allylic Amination of Substituted Monodonor Diazaphospholidine Ligands”, Christopher W. Edwards, Mark R. Shipton, Nathanial W. Alcock, Howard Clase and Martin Wills, Tetrahedron, 2003, 59, 6473-6480.
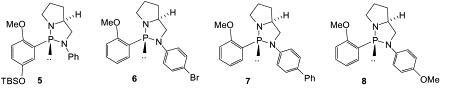
65) ‘‘Synthesis and Preliminary Studies on a Novel Class of Soluble Amino Alcohol Reagents Based on Methacrylate Copolymers’ Christopher W. Edwards, David M. Haddleton, David Morsley, Mark R. Shipton and Martin Wills, Tetrahedron, 2003, 59, 5823-5830.

64) 'Studies of Intramolecular Alkylidene Carbene Reactions; a Approach to Heterocyclic Nucleoside Bases', Gerard Hobley, Keith Stuttle and Martin Wills, Tetrahedron, 2003, 59, 4739 - 4748.
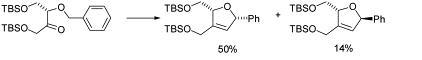
63) ‘Synthesis and hydrolysis studies of a peptide containing the reactive triad of serine proteases with an associated linker to a dye on a solid phase support’, David J. Morris, John M. Clough, Raymond V. H. Jones, Hannah McCann and Martin Wills, Org. Biomol. Chem., 2003, 1, 1486 - 1497.
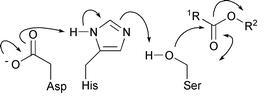
62) ‘Enantioselective synthesis of aziridines using asymmetric transfer hydrogenation as a precursor for chiral derivatives used as bonding agent for rocket solid propellants’, Aparecida M. Kawamoto and Martin Wills, Quimica Nova, 2002, 25, 921-925.
61) “Dynamic Kinetic Resolution – Asymmetric Transfer Hydrogenation of 1-Aryl Substituted Cyclic Ketones”, Nathanial J. Alcock, Inderjit Mann, Philip Peach and Martin Wills, Tetrahedron: Asymmetry, 2002, 13, 2485-2490.
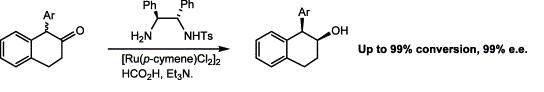
60) “Synthesis of 2,5-dihydrofurans via alkylidene carbene insertion reactions”, Ahmed Bourghida, Louise F. Walker, Stephen Connolly and Martin Wills, J. Chem. Soc., Perkin Trans. 1 2002, 965 - 981.

59) “Asymmetric Reduction of Cyclic Enones to Allylic Alcohols”, Jerome Hannedouche, Jennifer A. Kenny and Martin Wills, Synlett, 2002, 263-266.
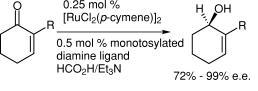
58) “Asymmetric Transfer Hydrogenation of Ketones”, Jennifer A. Kenny, Matthew J. Palmer, Tim Walsgrove, Aparecida M. Kawamoto and Martin Wills, J. Chem. Soc., Perkin Trans. 1., 2002, 416-427.
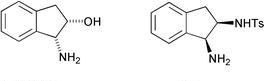
57) “An efficient method for the synthesis of N,N’-dimethyl-1,2-diamines”, Heather Tye, Colin Eldred and Martin Wills, Tetrahedron Lett., 2002, 43, 155-158.

56) “Influence of substitution pattern on intramolecular alkylidene carbene insertion reactions”. Ahmed Bourghida, Vince Wiatz and Martin Wills, Tetrahedron Lett., 2001, 42, 8689-8692.
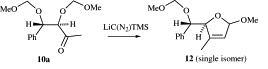
55) “Synthesis and Applications to Asymmetric Catalysis of a series of mono- and bis(diazaphospholidine) ligands", Donald Smyth, Heather Tye and Martin Wills, J. Chem. Soc., Perkin Trans 1. 2001, 2840 - 2849.
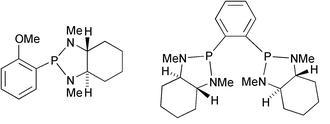
54) "Synthesis of Benzazaphospholidine Ligands via an Intramolecular Cyclisation Reaction”, Wilson Leung, Sarah Cosway, Raymond V. H. Jones, Hannah McCann and Martin Wills, J. Chem. Soc., Perkin Trans 1, 2001, 2588-2594.
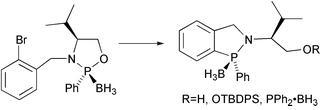
53) “Rhodium versus ruthenium: contrasting behaviour in the asymmetric transfer hydrogenation of a-substituted acetophenones”, David J. Cross, Jennifer A. Kenny, Ian Houson, Lynne Campbell, Tim Walsgrove and Martin Wills, Tetrahedron: Asymmetry, 2001, 12, 1801-1806.
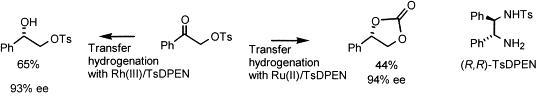
52) “Enantioselective Synthesis of b-Hydroxy Amines and Aziridines using Asymmetric Transfer Hydrogenation of a-Amino Ketones”, Aparecida M. Kawamoto and Martin Wills, J. Chem. Soc., Perkin Trans 1, 2001, 1916-1928.
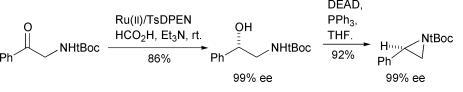
51) “Palladium-Catalysed Tandem Reactions to form 1-Vinyl-1H-Isochromene Derivatives”, Roger Mutter, Ian B. Campbell, Eva M. de la Neva, Andrew T. Merritt and Martin Wills, J. Org. Chem., 2001, 66, 3284-3290.
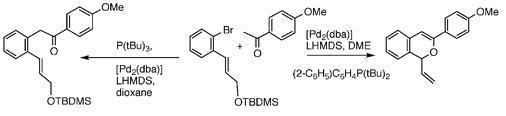
50) “Modification and inhibition of Vancomycin-group antibiotics by formaldehyde and acetaldehyde’, Albert J. R. Heck, Pauline J. Bonnici, Eefjan Breukink, David J. Morris and Martin Wills, Chemistry; Eur J., 2001, 7, 910-916.
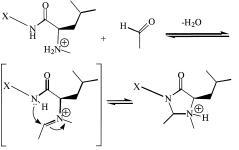
1996-2000
49) ‘Enantioselective synthesis of b-hydroxy amines and aziridines using asymmetric transfer hydrogenation of a-amido ketones’, Aparecida M. Kawamoto and Martin Wills, Tetrahedron: Asymmetry, 2000, 11, 3257-61.

48) “Direct formation of 1-vinyl-1H-isochromene derivatives via a palladium-catalysed coupling reaction”, Roger Mutter, Eva M. de la Neva and Martin Wills, Chem. Commun., 2000, 1675-6.
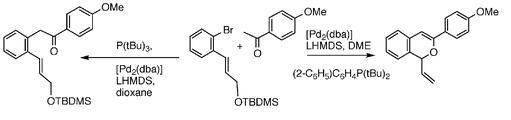
47) “Rapid Assembly and Synthetic Applications of a Supported Poly-a-amino acid containing Phosphine Groups”, Christopher W. Edwards, Mark Shipton and Martin Wills, Tetrahedron Lett., 2000, 41, 8615 -8619.
46) “Rhodium-Mediated Asymmetric Hydroformylation using a Novel Bis(Diazaphospholidine) ligand: Simon Breeden, David J. Cole-Hamilton, Douglas F. Foster, Gary J. Schwarz and Martin Wills, Angew. Chem., Int. Edn., 2000, 39, 4106-9.
45) “Electrospray analysis of transfer hydrogenation reactions”, Jennifer A. Kenny, Kees Versluis, Albert J. R. Heck, Tim Walsgrove and Martin Wills, Chem. Commun, 2000, 99-100.
44) “Recent Developments in the area of Asymmetric Transfer Hydrogenation”, Martin Wills, Matthew J. Palmer, Athene R. C. Smith, Jennifer A. Kenny, Tim Walsgrove Molecules, 2000, 5, 1-15. WRAP. Open access from Molecules website.
43) “ESPHOS and SEMIESPHOS: A New Family of Monodonor and Bidentate Diazaphospholidine Ligands for Asymmetric Catalysis”, Martin Wills and Simon W. Breeden, J. Org. Chem., 1999, 64, 9735-9738.
42) “Synthesis and Electrospray-MS Studies on a Chiral, non-Racemic, Phosphoramide Receptor Molecule”, Athene R. C. Smith, Jennifer A. Kenny, Albert J. R. Heck, J. Jantien Kettenes-van der Bosch and Martin Wills, Tetrahedron: Asymmetry, 1999, 10, 3267-70.
41) “Asymmetric Transfer Hydrogenation of a-Amino and a-Alkoxy Substituted Ketones” Jennifer A. Kenny, Matthew J. Palmer, Athene R. C. Smith, Tim Walsgrove and Martin Wills, Synlett, 1999, 1615.
40) Martin Wills, Mark Gamble, Matthew Palmer, Athene R. C. Smith, John R. Studley and Jennifer A. Kenny “Novel Catalysts for the Asymmetric Reduction of Carbonyl Groups” J. Mol. Catal. A; Chemical, 1999, 146, 139-148. (a paper resulting from an invited lecture at the “9th International Symposium on Relations between Heterogenous and Homogenous Catalysis”, Southampton, July 1998).
39) “The Use of Phosphinamide N-Protecting Groups in the Diastereoselective Reduction of Ketones”, Matthew J. Palmer, John R. Studley, Tim Walsgrove and Martin Wills, Tetrahedron, 1998, 54, 8827.
38) “A Novel Phosphinamide Catalyst for the Asymmetric Reduction of Ketones by Borane". Mark P. Gamble, Athene R. C. Smith and Martin Wills, J. Org. Chem, 1998, 63, 6068.
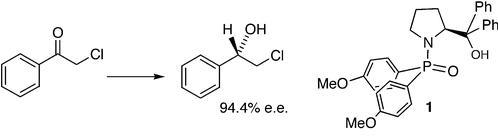
37) "Formation of 2,5-Dihydrofurans via Alkylidene Carbene Insertion Reactions", Louise Walker, Stephen Connolly, and Martin Wills, Tetrahedron Lett, 1998, 39, 5273 .
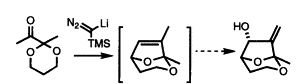
36) “Chiral Phosphinamides: New Catalysts for the Asymmetric Reduction of Ketones by Borane”, Barry Burns, N. Paul King, Heather Tye, John R. Studley, Mark Gamble and Martin Wills, J. Chem. Soc., Perkin Trans. 1, 1998, 1027
35) "Design, Synthesis and Preliminary Studies on a Novel Class of Chiral Receptor for the recognition of Amino Acid Derivatives", Heather Tye, Colin Eldred, Mary F. Mahon and Martin Wills, J. Chem. Soc., Perkin Trans. 1, 1998, 457.
34) "An Efficient New Ligand for the Asymmetric Catalysis of Transfer Hydrogenation of Ketones", Matthew Palmer, Tim Walsgrove and Martin Wills, J. Org. Chem., 1997, 62, 5226.
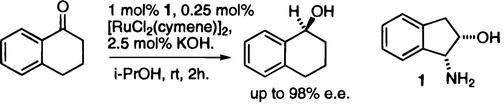
33) "Synthesis, and Applications of a New Class of Phosphorus Donor Ligands for Asymmetric Catalysis", Heather Tye, Donald Smyth, Colin Eldred and Martin Wills, J. Chem. Soc., Chem. Commun., 1997, 1053.
32) "The Total Synthesis of Halicholactone and Neohalicholactone", Douglas J. Critcher, Stephen Connolly and Martin Wills, J. Org. Chem., 1997, 62, 6638.
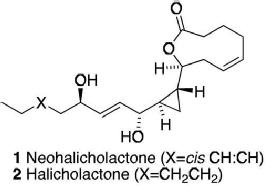
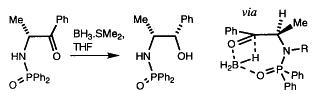
31) "The Use of Phosphinamide N-Protecting Groups for the Diastereoselective Reduction of Ketones", Matthew Palmer, John R. Studley, Tim Walsgrove and Martin Wills, Tetrahedron Lett., 1997, 38, 2315.
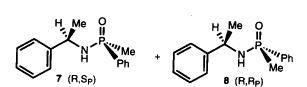
30) "Phosphinamides Catalysts Containing a Stereogenic Phosphorus Atom for the Asymmetric Reduction of Ketones by Borane", Barry Burns, Mark P. Gamble, Athene R. C. Simm, John R. Studley Nathaniel W. Alcock and Martin Wills, Tetrahedron: Asymmetry, 1997, 8, 73.
29) "Design, Synthesis and Applications of a Ketone Reduction Catalyst Containing a Phosphinamide Combined with a Dioxaborolidine Unit", Mark P. Gamble, John R. Studley and Martin Wills, Tetrahedron: Asymmetry, 1996, 7, 3071.
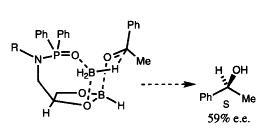
28) "Synthesis and Applications of a New Class of C2 Symmetric Phosphorus Donor Ligand for Asymmetric Catalysis", Guy Brenchley, Michael Fedouloff, Eric Merifield and Martin Wills, Tetrahedron: Asymmetry, 1996, 7, 2809.
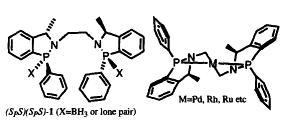
27) "New Chiral Phosphinamide Catalysts for Highly Enantioselective Reduction of Ketones", John R. Studley, Mark Gamble and Martin Wills, Tetrahedron Letters, 1996, 37, 2853.
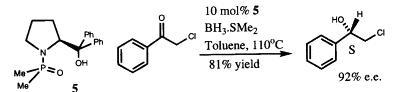
26) "The Asymmetric Synthesis of Amines using a Chiral, non-Racemic, Benzylidene Sulphinamide Derived from a Recyclable Precursor", David R. J. Hose, Mary F. Mahon, Kieran C. Molloy, Tony Raynham and Martin Wills, J. Chem. Soc., Perkin Trans. I., 1996, 691.
25) "Recoverable Chiral Sulphoxides for Asymmetric Synthesis: Application to Stereoselective Carbonyl Reduction and the Asymmetric Synthesis of Allylic Alcohols", Roger J. Butlin, Ian D. Linney, Mary F. Mahon, Heather Tye and Martin Wills, J. Chem. Soc., Perkin Trans. I., 1996, 95.
1990-1995
24) "New ligands for Asymmetric Palladium Catalysed Allylic Substitution Reactions. Synthesis and X-ray Crystal Structures of Two Enantiomerically Pure Dihyrobenzazaphosphole-Borane Complexes", Guy Brenchley, Michael Fedouloff, Mary F. Mahon, Eric Merifield, Kieran C. Molloy and Martin Wills, Tetrahedron, 1995, 51 10581.
23) "Synthesis of a Synthetic Receptor via Directed Lithiations of Dibenzofuran", Heather Tye, Colin Eldred and Martin Wills, Synlett, 1995, 770.
22) "The Total Asymmetric Synthesis of Halicholactone and Neohalicholactone", Douglas J. Critcher, Stephen Connolly and Martin Wills, Tetrahedron Lett., 1995, 36, 3763.
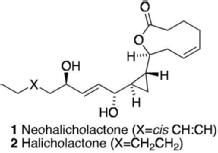
21) "Synthesis and X-ray Crystal Structure of the Right Hand Hemisphere of Halicholactone and Neohalicholactone", Douglas J. Cricher, Stephen C. Connolly, Mary F. Mahon and Martin Wills, J. Chem. Soc., Chem. Commun., 1995, 139.
20) "The Effect of Zinc(II)Bromide on the Reduction of a Chiral, non-Racemic, Benzylidene Sulphinamide Derived from a Recyclable, Cyclic Sulphinamide", David R. J. Hose, Tony Raynham and Martin Wills, Tetrahedron Lett, 1994, 35, 5303.
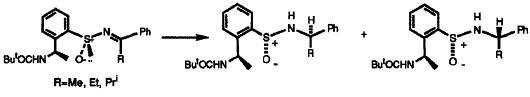
19) "Stereochemical Requirements for a New Class of Asymmetric Ketone Reduction Catalysts Containing an N-P=O Structural Unit", Barry Burns, N. Paul King, John R. Studley, Heather Tye and Martin Wills, Tetrahedron: Asymmetry, 1994, 5, 801.
18) "A New Class of Chiral Phosphorus Catalyst for Asymmetric Palladium Catalysed Allylic Substitution Reactions", Guy Brenchley, Eric Merifield, Martin Wills and Michael Fedouloff, Tetrahedron Lett., 1994, 35, 2791.
17) "The Asymmetric Synthesis of Allylic Alcohols Using a Recoverable Chiral Sulphoxide", Ian D. Linney, Heather Tye, Martin Wills and Roger J. Butlin, Tetrahedron Lett., 1994, 35, 1785.
16) "New Catalysts Containing an N-P=O unit for the Asymmetric Reduction of Ketones". Barry Burns, John R. Studley and Martin Wills, Tetrahedron Lett, 1993, 34, 7105.
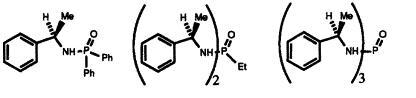
15) "Synthesis of a new class of Asymmetric Ketone Reduction Catalyst via a Diastereoselective Cyclisation Reaction: X-Ray Crystal Structure of S(P)R-(-)-N-(tert-Butyldiphenylsilyl)dihydrobenzazaphosphole oxide.", Barry Burns, Eric Merifield, Mary F. Mahon, Kieran C. Molloy and Martin Wills, J. Chem. Soc., Perkin Trans. 1, 1993, 2245.
14) "Asymmetric Synthesis of Amines using a Chiral, Non-Racemic, Cyclic Sulphinamide", David R. J. Hose, Tony Raynham and Martin Wills, Tetrahedron: Asymmetry, 1993, 4, 2159.
13) "Studies of the Zinc(II) Catalysed Addition of Silyl Enol Ethers to Cyclic Enol ethers", John R. Studley and Martin Wills, J. Organomet. Chem., 1993, 455, C3-C5.
12) "Recoverable Chiral Sulphoxides for Asymmetric Synthesis: Preparation, Regeneration and Application to the Asymmetric Aldol Reaction", Roger J. Butlin, Ian D. Linney, Douglas J. Critcher, Mary F. Mahon, Kieran C. Molloy and Martin Wills, J. Chem Soc., Perkin Trans. 1, 1993, 1581.
11) "A New Class of Recoverable Chiral Sulphoxide: Application to the Asymmetric Synthesis of b-hydroxy esters" Martin Wills, Roger J. Butlin and Ian D. Linney, Tetrahedron Lett., 1992, 33, 5427.
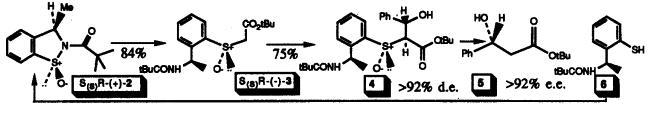
10) "Dependence of the Stereoselectivity of Ring Closure on the Nature of the Leaving Group: Application to the Synthesis of a New Class of Chiral Sulphoxide for the Control of the Aldol Reaction" Martin Wills, Roger J. Butlin, Ian D. Linney and Richard W. Gibson, J. Chem. Soc., Perkin Transactions I, 1991, 3383-3385.
9) "Chiral Recognition Reactions of Homochiral Sulphinate Esters: A Study of the Reaction Between the Enolate, and the Anion of the Corresponding Hydrazone, Derived from 4-t-Butyl Cyclohexanone and (1R,2S,5R)-(-)-Menthyl-(S)-p-Tolylsulphinate", Martin Wills, Ian D. Linney, Christopher Lacy, Mary F. Mahon and Kieran C. Molloy, Synlett., 1991, 836-840.
Postdoctoral Research (Lead author is W. Oppolzer)
8) (Prepared by C. Chapius) "X-ray Crystallographic Structure of Two Sultam Chiral Auxiliaries", Christian Chapius, Gerald Bernardinelli, Arend J. Kingma and Martin Wills, Helv. Chim. Acta., 1997, 80, 1607.
7) "Chiral Toluene-2,a-Sultam Auxiliaries; Asymmetric Diels-Alder Reactions of N-enoyl Derivatives", Wolfgang Oppolzer, Martin Wills, Martha J. Kelly, Marcel Signer and Julian Blagg, Tetrahedron Lett., 1990, 31, 5015.
6) "Chiral Toluene-2,a-Sultam Auxiliaries; Preparation and Structure of Enantiomerically pure (R)- and (S)-Ethyl-2,1'-sultams", Wolfgang Oppolzer, Martin Wills, Christian Starkemann and Gerald Bernardinelli, Tetrahedron Lett., 1990, 31, 4117.
Postgraduate research work (Lead author is S. G. Davies)
5) "Chiral Recognition in the Reaction of the Enolate Derived from [(C5H5)Fe(CO)(PPh3)COCH2OCH2Ph] with Chiral Secondary Halides", Stephen G. Davies, David Middlemiss, Alan Naylor and Martin Wills, J. Chem. Soc., Chem. Comm., 1990, 797.
4) "Application of the Iron Acyl Complex R-(-)-[(C5H5)Fe(CO)(PPh3) COCH2O({1R,2S,5R}Menthyl)] as a Homochiral Formyl Anion Equivalent", Stephen G. Davies, David Middlemiss, Alan Naylor and Martin Wills, Tetrahedron Lett., 1989, 30, 2971.
3) "Chiral recognition in the Reaction of the Enolate Derived from [(C5H5)Fe(CO)(PPh3)COCH2OCH2Ph] with trans- and cis-2,3-epoxybutane; Application to the Asymmetric Synthesis of cis-trans- bg-Disubstituted-g-Lactones", Stephen G. Davies, David Middlemiss, Alan Naylor and Martin Wills, Tetrahedron Lett., 1989, 30, 587.
2) "Stereospecificity of the Rearrangement of the a-Alkoxy Iron Acyl Complex [(C5H5)Fe(CO)(PPh3)COCH2OCH2Ph] to the a-Metalla Ester [(C5H5)Fe(CO)(PPh3) CH2CO2CH2Ph]", Stephen G. Davies and Martin Wills, J. Chem. Soc., Chem. Commun., 1987, 1647.
1) "An Approach to the Asymmetric Synthesis of a-Hydroxy Acids", Stephen G. Davies and Martin Wills, J. Organomet. Chem., 1987, 328, C29-C3.
